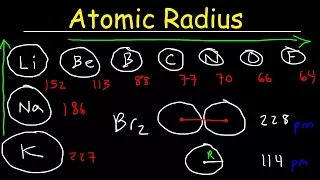
Ionization Energy Electron Affinity Atomic Radius Ionic Radii Electronegativity Metallic Character
May 30, 2021valence
electron
s, so this element has two valenceelectron
s, so we have to figure out which one of these. the elements contain two valence electrons sulfur is found in group six a so sulfur has six valence electrons arsenic is found in group five it has five valence electrons silicon has four and gallium has three magnesium has two and potassium has one then the answer is magnesium because it contains two valence electrons, so the third electron that is removed from magnesium is a central electron that is associated with this number 7730. Now the next topic of interest is the Electronicaffinity
.Electron
affinity
is associated with theenergy
change that occurs when an electron is added to a gaseous atom. now when you add electrons to non-metals that really want electrons, particularly electronegative non-metals, they tend to release a lot ofenergy
and every time energy is released in a reaction, you have an exothermic reaction, which is why chlorine has a strong desire of electrons when it acquires an electron, it becomes. into chloride and therefore releases a lot of energy, so the energy released in a reaction is associated with the electronic affinity of the element. Ionization energy is the opposite.
More Interesting Facts About,
ionization energy electron affinity atomic radius ionic radii electronegativity metallic character...
Ionization energy is the energy required to remove an electron from a gaseous atom, while electron affinity is the energy. required or the energy that the energy change that occurs when an electron is absorbed by a gaseous atom it is important to know that halogens are the most exothermic in terms of electron affinity because they are so electronegative that they really want electrons, so if you add an electron , say a gaseous fluorine atom, when converted to fluoride, will release negative 327.8 kilojoules per mole, making it highly exothermic. The fact that it releases so much energy means that as it acquires that electron it becomes very stable, so if the addition of an electron to a gaseous atom produces a stable ion it will be highly exothermic, if it produces something unstable it is likely that is not very exothermic, it could even be endothermic, so what are the electron affinity trends, generally speaking, keyword generally electron? the affinity increases or becomes more exothermic as you go to the right now there are many exceptions it is useful to know these numbers 76 45 13 28 what does that mean?
These are group numbers. Group 7 is the most exothermic group when you add an electron. An element in group seven, the halogens, will release the most energy. Group six is next and then there is group four, but not five. Four is more exothermic than five and then one, three, two and eight are the least exothermic, in fact the most. The elements in two and eight are endothermic, you have to put in energy to add an electron. Now it's easy to see why group seven is the most exothermic because they are the most electronegative, they only need one electron to complete their oxide, but why is it group four? more exothermic than group five, for example, the carbon that is in group four is more exothermic than, say, the nitrogen that is in group 5a.
Now carbon has the configuration that ends in 2p2 for nitrogen, it is 2p3, so if we add one electron to carbon and another to nitrogen, which one is going to be more stable currently carbon has two electrons in its 2p orbital nitrogen has three, so if we want to create the c minus gas ion we just need to add one electron to the carbon and notice that the orbitals are the two p orbitals are still unpaired, so because you have this empty space for an extra electron, the c minus ion is quite stable. Now what about adding an electron to an element in group 5a like nitrogen?
Once we add it, notice that there will be electron repulsion between the two. electrons in this orbital, so this is not a good stable arrangement and it is due to electron repulsion and this is why group 4a elements are more exothermic than group 5a elements, when you add an electron to carbon , you can or will create a fairly stable ion, but when you add it to nitrogen, the ion will not be as stable due to electron repulsion and that is why Group 4a elements are more exothermic than Group 5a elements in terms of electron affinity. . Now what about group 1 versus group 2?
Look at the numbers 76 45 13 28. If you add an electron to a group one element, it will be exothermic, but if you add it to a group two element, most of them are endothermic, so let's understand why we use sodium and magnesium as examples, so sodium's configuration ends in 3s1, magnesium is 3s2, so magnesium has two paired electrons and sodium only has one. The next orbital after 3s is 3p, so if we add an electron to sodium, that electron will go in this empty 3s orbital or this half-filled 3s orbital, but if we add an electron to magnesium, we have to put that electron in a higher 3p orbital, it is more stable. putting an electron in the lower energy level than in a higher energy level, even if there will be some kind of electron repulsion, by adding the electron in this half filled orbital, the electron affinity for sodium is still relatively exothermic, It is much smaller than four and five, but it is still more exothermic than group two.
Now for group two elements, it is endothermic because we are putting an electron at a higher energy level and therefore we have to add energy to put that electron there, and that is why it is endothermic for many members of the group. 2 elements and the same is true for group a elements for example let's consider that neon is in group 8 and ends in 2p6 so the 2p orbital is completely filled to add an electron we need to add it to the 3s orbital and so, because we are adding an electron to a higher energy level, it is going to be endothermic, we need to add energy to the system to add that electron to that atom, that is why group two and group eight are endothermic with respect to affinity electronics because its electrons Can electrons in its configuration be completely full?
They are completely paired, so to add a new electron you must put it at a higher energy level. All other groups 7 6 4 5 1 and 3 you don't have to add an electron to a higher energy level, you just have to add it to an empty or half-filled orbital, so adding electrons to half-filled or empty orbitals is usually an exothermic process, but putting an electron and a higher energy level is usually an endothermic process. process, so when it comes to electron affinity, you have to ask yourself if I add this electron, will it create a stable ion or an unstable ion?
Is there room for me to put this electron somewhere? Is there a half full or empty orbital? to put it somewhere, if there is it will probably be exothermic, if you have to put it at a higher energy level then it will probably be endothermic, so I hope these principles help you understand the concept of electron affinity and when . will be endothermic versus when will it be exothermic now let's try one more problem, this will be the last problem in this video, so let's say that if we have the following elements chlorine, phosphorus, argon, magnesium, sodium and silicon, classify the following elements in terms of your electro, I mean your electron affinity values, so you want to classify them from endothermic to most exothermic.
Now it's helpful to know the order 76 45 13 28, so as you travel this way towards group 7, it's going to be mostly exothermic towards groups 2 and 8. It's going to be endothermic, so chlorine is in group 7, phosphorus is in group five, argon is in group eight, magnesium is in group two, sodium is in group one, silicon is in group four, so from endo to exo let's start with group eight, so argon goes to be endothermic and magnesium, which is in group two, that's endothermic, now we don't have any group three elements, so we can get rid of that, next is group one , which is sodium and then we have group five, which is phosphorus. and then group four, which is silicon, we do not have any group 6 elements, the last one is group 7, which is chlorine, so of the elements listed, if we add an electron, chlorine will be the most exothermic that is will release. a lot of energy while argon will be the least exothermic or the most endothermic to add an electron to a gaseous argon atom we need to add energy for that to work so that's it for this video thanks for watching and have a great day
If you have any copyright issue, please Contact