Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6
Jun 01, 2021The water will balance the hydrogen. 22 hydrogen atoms on both sides. And finally, oxygen. Since we have 12 carbon dioxide molecules and 11 water molecules in the resulting materials, we have 35 hydrogen atoms. If you look at the reactants on the left, you will see that you have 11 oxygen atoms in the sucrose molecule and two in the oxygen molecule. The carbon and hydrogen are balanced by a sucrose molecule, so let's leave it at that. But the number of interacting oxygen molecules can increase. Since you know that you need 25 atoms and that there are 11 in sucrose, you need the remaining 24 atoms, which are equivalent to 12 oxygen molecules.
The reaction is now balanced and you know exactly what my body produces. For each molecule of sucrose that is metabolized, I have to inhale 12 molecules of oxygen and in return, in addition to the effect of sugar, I will produce 12 molecules of carbon dioxide and 11 molecules of water. This is of great benefit to understanding the ratios of chemicals as they interact at the molecular level. Whether in a laboratory or in life, we have to work with a number of materials that can be measured. So the last trick you need to calculate reactants is to calculate specific amounts of reactants and products.

More Interesting Facts About,
stoichiometry chemistry for massive creatures crash course chemistry 6...
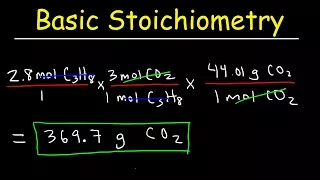
For example, how much oxygen do I have to inhale to burn five grams of sugar? To know this we have to focus on the left side of the equation, because we only have to determine the amount of reactants. First, we convert the balanced equation to molar masses. To go from molecules to grams, we must first go through the moles. When we calculated the molar masses, we found that the ratio of sucrose to oxygen is similar, 394 grams of oxygen for every 343.3 grams of sucrose. We then compare this ratio to the masses of the reactants in the experiment.
Five grams of sugar with x of oxygen, I hope you know how to find the value of x. For every five grams of sugar I digest, I inhale 5.6 grams of oxygen, and I know that's 35 inhalations. So if I survive the next minute and a half, I'll burn off these five grams of sugar. Health! Today we learned about two of the most important chemical units of measurement, the atomic mass unit and the mole. We also learned how to calculate molar mass and balance chemical equations. We also talked about using molar ratios to calculate the amount of things going into and out of a reaction.
Thank you for watching this episode of Crash Course Chemistry, which was filmed, edited and directed by Nick Jenkins. This episode was written by Blake DeBastino and edited by Dr. Heiko Langner. The sound designer is Michael Aranda and the graphic team is Thought Café.
If you have any copyright issue, please Contact