Mole Ratio Practice Problems
May 30, 2021Okay, now I'm going to solve a bunch of molar relationship
problems
so you can understand them. They are very important for stoichiometry, for understanding stoichiometry. Okay, I'll do each problem two ways. First I'm going to treat the equation, the chemical equation, as a kind of recipe, where we have our ingredients and then we have the step where we are baking. This method really makes sense, as you will do. I understand what you're doing, but it requires a bit of skill, then I'll solve each one, probably using a conversion factor method. The conversion factor method does not require any thought, but it does not make any sense, so it is very easy to achieve.I have a habit of only using conversion factors in calculations, but I have absolutely no idea what you're actually doing or why you're doing it right, which is why I'm going to solve each of these
problems
in two different ways, right? OK? Let's start here is the first equation. I am going to work with twomole
s of water. Water produces twomole
s of hydrogen and oxygen gas. Here, if there is no coefficient versus oxygen versus one of these chemicals, we know what it is. really one that means a mole, so hey, if it helps you, go ahead and write that one down.There is our equation. Here is the question: how many moles of O2 of oxygen will be produced from six point two moles of water? Well, first of all I want to You have to think of this as a recipe and right now it says we start with two moles of this and that gives us two moles of this and one mole of this, but we're not talking about starting with two moles of water. talking about starting with 6.2 moles of water, okay, so this is like when you're cooking and you have a double, triple or quadruple recipe, what do we have to do with this recipe so that instead of starting with two moles of water we start with six point two moles of water, okay, we have to multiply everything in this equation by something that will give us 6.2 moles of water, we can solve this with a couple of lists, the first thing I can do is 6.2, which is this divided by two which is that and that gives me 3.1 well that gives me the factor and what I mean by that is we take each of these numbers and we multiply it by three point one two times three point one and now we get six point two moles of this, okay, now we start with six point two instead of two, we multiply this by 3.1.
Now we also want to multiply the number of H two by three point one, so now we get. six point two moles of H 2 and we want to take this number, which is one times three point one, we multiply it and we get three point one moles of O2, so once again we treat this as a cooking recipe and we are doubling or tripling in Instead of multiplying by two or by three or by 4 we multiply everything in this recipe by 3.1 we get six point two here so that's what we start with then we get six point two moles of h2 and we get three point one moles of O2 and that makes sense, because There is a two to one
ratio
between water and oxygen.We start with two of these and we get one of these, so this is half of what we have here. Well, if we start with six point two. we multiply everything by three point one and we get six point two here and this three point one is half of what we had here, okay, this is how we can do this equation, treating it like a recipe in the kitchen, now let's see how I can do this using conversion factors. Well, what we're going to do is start with six point two moles of water. Now what I want to do is write a conversion factor that tells me the relationship between the moles. of water and the moles of oxygen, okay, we can summarize it with this that tells us, wherever two moles of h2o get one mole of o2, two here one here, okay, we get this relationship and what I can do is use this To write a conversion factor, there are two different conversion factors that I can get from this.
I can write two moles of h2o over one mole of o2, okay, that's one or I can reverse it. I can make one mole of o2 over two moles, so of course. I'm just getting the chemical equation numbers two and one, so I have these two conversion factors that are inverted. I could use any of them, both are equally good, but the one I want to choose is the one. which I can multiply by this and cancel moles of h2o, okay, that's going to be the one with h2o at the bottom because it's up here, so I'm not going to use this one, I'm going to use this one.
I'm going to multiply it here and now I have h2o at the top h2o at the bottom so they cancel out and when I do these calculations I'm going to do six point two times one divided by two equals three point one moles of o2 and Check it out. I use a conversion factor method and get exactly the same response I got when using this as a recipe. Okay, so the recipe or the conversion factor gives the same number. Okay, let's move on to the next one. Well, how? It will take many moles of h2o to produce 19.2 moles of o2.
Let's treat this as a recipe first. Well, right now this is a recipe for one mole of O2. What are we going to have to do with this recipe? Double, triple, quadruple is what I mean, what are we going to have to do with this recipe to make it a nineteen point two mole of O2 recipe? Well, here's one mole of O2 in the recipe, so we shouldn't be surprised that we have to multiply the recipe by nineteen point two and now if we multiply it by nineteen point two it will become a recipe of nineteen point two oh two times nineteen point two and this is going to give me thirty-eight point four moles of h2 multiply this by nineteen point two because just like when you are cooking well you have to multiply everything in the recipe by the same number and this will give us thirty-two. eight point four moles of water, this is what happens to the recipe when we size it up to cook for nineteen point two moles of o2 then the answer is how many moles of h2 are there going to be are we going to need thirty eight point four moles of h2o that's the recipe method okay now let's look at the conversion factor method like before we realize the relationship between water and oxygen using the chemical equation here are two of these two one two of these so we can get these two conversion factors yeah, one mole of o2 to two moles of h2o or two moles of h2o to one mole of o2 I'm going to start here with nineteen point two moles of o2 and I want to be able to multiply this by the conversion factor that will cancel out my moles of o2, so I'm going to choose this one here because it has moles. of o2 at the bottom cancel cancel I do 19 point two times 2/1 equals thirty-eight point four moles of h2o I know it's moles of h2o because that's the unit that fits me so sometimes people ask for what do you do?
Both ways, why are you doing the absolution with the recipe and then with the conversion factor? Okay, the reason is that the recipe makes a lot more sense to me, but teachers and textbooks are in love with conversion factors, they love them, and they can't get them. Enough, I don't think conversion factors make any sense, but just because they are commonly used, your teachers will ask you to do it, your textbooks will ask you to do it, that's why I'm doing them. too, but what I want you to focus on is why we're doing it the way we multiply things and get the answers here by pretending it's a recipe.
I want you to focus on this and understand what is happening as much as you can. Look, the answer we get from the conversion factor method is the same one we get when we treat it as a recipe to multiply everything by some number, okay, let's move on to the next question, so two moles of h2s. hydrogen sulfide combined with three moles of oxygen o2 to get two moles of so2 and two moles of h2o using this equation, we are asked how many moles of o2 are needed to combine with 8.4 moles of h2s, what do we have to do with our recipe to cook with 8.4 moles of h2 s instead of two moles of h2 s what we are going to want to do is multiply this by some number that will give us 8.4 how do we know what that is?
You might be able to do it in your head or you can do 8.4 divided by 2 and that will give us four point two, so 4.2 is the number that we're going to have to multiply everything by in order to move. from two moles of this to a recipe that uses 8.4 moles of this good, so we are going to multiply everything by 4.2 good, here we have three times 4.2 and that will give us 12 point 6 moles of o2 to two 38.4 to twelve point six, good , let's see how we can do this using conversion factors. We'll start with eight point four moles of h2s and multiply it by one of these two conversion factors.
These conversion factors just tell us the
ratio
that we have here of two moles of h2 s to three moles of o2 and I can use this ratio to make these two conversion factors, which one do I want to use? I want to use the one with h2s at the bottom so that it cancels, so I choose this one cancels F cancels and this is going to give me a surprise: twelve point six moles of o2 one mole of o2 because it is the unit that remains here, okay, this is how we can do it, treating it as a recipe, this is how we can do it using conversion factor here is an XML ratio problem look, maybe you are already getting used to this, maybe you feel great, turn off the video and move on to the next thing, but if you still like something orpractice
, we are going to party all night with molar relationship problems we will keep doing them until you feel really comfortable and you can and are sure that you would like to answer any questions that arise. present to you, okay, starting with 9.2 moles of o2, how many moles of h2s will you need and how? how many moles of so2 will you be okay, so starting with 9.2 moles of o2 right now, our equation is written to start with three moles of o2, so what are we going to have to do?We have to figure out how to double, triple, quadruple whatever we want. Do this to increase it from three moles of O2 to nine point two moles of objects. Why would I have to multiply it to turn this 3 into 9.2? I can solve it by doing nine point two divided by three. and I'm going to get three point one, so three times three point one will give me nine point two. I'm a little round here, okay, if you do the math, it's not exactly right, okay, multiplied by three points once. Give me nine point two moles of o2 and how many moles of h2 s will I need?
Well, I'm going to have to multiply everything in the equation by the same amount, so multiplied by three point one here will give me six points. two moles of h2 s, so that's how much h2 I'm going to need to combine with my nine points, two moles of o2 and then how many moles of so2 will I get? I take my two moles of so2 and multiply them by three points. one I also have to do the same thing the whole equation is when I get six point two moles of so2 this is how we do it let's see how we do the conversion factors you guys are probably already mastering this now so I'm not going to show all the steps from the conversion factor we start with nine point two moles of o2 multiplied by what is our relationship between Oh two and h2s we have three moles of o2 - two moles of h2 s so I want to write that as a conversion factor with o2 at the bottom so that it cancels out, so three moles of o2 - two moles of h2 s moles of oxygen up here cancel a little action down there cancel and I get the same answer that I got here. get nine point two moles of h2s and now let's see how we can do this with the moles of so2, it will be exactly the same.
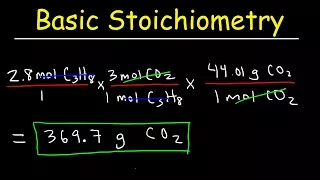
I'm not even going to like getting rid of this, everything is canceled here. I'm going to multiply it by the equation which shows that it's a conversion factor that relates oxygen here and so2 here, okay, again three moles of this times two moles of this, so three moles of o2 goes on the bottom and two moles of so2 go on top, cancel this out. It's like this is canceling that and I'm going to get after I do the calculations nine point two times two divided by three is equal to six point two moles of so2 sometimes I write moles or knees sometimes ml well because sometimes it gives me laziness Well, that's it, let's do two more, if this feels good, turn it off and move on, so here we have an equation for burning propane, which is c3h8, nothing in front of this, so we can put a 1 here , one mole of this plus five moles. of o2 gives me three moles of co2 and four moles of h2o my question here asks me how many moles of oxygen are needed to react with 7.2 moles of propane what do we have to do with this equation to size it so that we are starting with 7, 2 moles of propane right now we're starting with one mole of propane okay so this isn't the hardest thing in the world we're going to have to multiply this whole equation by 7.2 and that's going to give us seven points. two moles of propane how many moles of oxygen are eaten well we have to multiply it by 7.2 also and that math is going to give me 36 point 0 moles of o2 let's look at the commercial factor method let's start with seven point out two moles of c3h8 and let's multiply that for a conversion factor that says one mole of C 3 H 8 times 5 moles of o2 that will be at the top that will be at the bottom this is at the top so we want this in a conversion factor to be at the bottom, so I say a mole.
I'm getting it from right here. one mole c3h8 below five moles. Oh, this cancels your top, thiscancels its lower part and gives us seven point two times 5/1 is equal to 36 point 0 moles of oxygen, okay, one more, the last one, how many moles of propane do I need to make thirteen point five moles of co2 to be able to do that ?A lot of O2 will be needed. What do we have to do with our equation right now to make it thirteen point five moles of CO2 instead of three moles of CO2? We have to multiply it by some number that moves it from three up. to thirteen point five and what I can do is do 13 point five divided by three and that will give me four point five so I can multiply this by four point five and that will turn my three into thirteen point five moles of co2 so when I speak How many moles of propane am I going to need, I multiply it by the same amount and I get one times four point five four point five moles of C 3 H five and then they ask me how much O2 am I going to need?
Well, I have to do the same with all the pieces in my recipe, so five times four point five will give me twenty-two point five moles of O2. This is what happens to my recipe when I increase. the size let me show you this real quick. I'm going to start with thirteen point five moles of co2 multiplied by a conversion factor that talks about the relationship between propane c3h8 and co2. Here the co2 will be at the bottom so that it cancels out. so three moles of co2 at the bottom one mole of c3h8 at the top 13.5 times 1/3 will give me four point five moles of c3h the number is the same even though the process is different Oh, what do I get? forgot?
Did I forget to cancel them or could I? I'm not going to get rid of them. I keep them there or to know how much O2 I will need. What is the relationship between CO2 and O2? It's 3 to 5. so 3 moles of co2 at the bottom and 5 moles of o2 at the top cancel 13.5 times 5/3 equals 22.5 moles of o2 the same answer regardless of whether I do it with the recipe method or the conversion factor method if you made it all the way to the end of this video, you're partying with me all night talking about molar ratios, you're a rock star, but more than anything, I bet Since this actually makes sense and I bet it can tackle any mole ratio problem you come across, feel free to use it. the recipe method or the conversion factor method, unless your teacher tells you that you have to use a conversion factor method, then go ahead and use it, but deep down you should know what is really happening and why you're multiplying by what we're doing and all that sir, very good, good luck
If you have any copyright issue, please Contact