Molarity Dilution Problems Solution Stoichiometry Grams, Moles, Liters Volume Calculations Chemistry
May 31, 2021In this video we will focus on marity and the
problems
associated with it, likedilution
problems
, conversion problems where you have marity, you need to find the mass ingrams
, themoles
, theliters
, things like that and then towards the end we go . to reviewsolution
s to geometry problems involving conversion from the marriage of one substance to another, double replacement reactions, precipitation reactions and thecalculations
associated with them, as well as limiting excess reactants, and even questions about the percentage of yog, so let's get started. to know that marity is a form of concentration marity represented by capital M ismoles
of solute divided byliters
ofsolution
so marity is moles overvolume
now sometimes you may need to find the moles, moles are equal to marity timevolume
marity you can represent it as moles per liter and if the volume is in liters you can see how the liters will cancel and you can get moles another equation you need to know is thedilution
equation M1 V1 is equal to M2 V2 the reason why this equation works is because the moles before adding water to dilute the solution are equal to the moles after adding water, now granted it has to be the moles of solute that are congruent because as add water the amount of the solution changes but the amount of solute remains the same if you are wondering what is the solute and what is the solvent, think about when you dissolve salt and water, salt is the solute, water is the solvent, but combined they form the solution, let's look at this problem 80 g of Sodium hydroxide is dissolved in enough water to produce a 500 M solution.What is the concentration of the solution? Let's find the concentration in marity marity is moles over liters now I'm going to solve it using the conversion, but our goal is to get moles The top liters are at the bottom, so let's start with what we have, which is 80 g of sodium hydroxide to convert from GRS to moles. We need the molar mass of NaOH according to the periodic table. The atomic mass of sodium is 23, that of oxygen is 16 and that of hydrogen. is one so this gives us a total of 40 G per mole so what this means is that 1 Mo of sodium hydroxide has a mass of 40 gr the molar mass tells you the number of
grams
that is equivalent to one mole of substance that now you want to set up in such a way that the unit grams cancel out, so now we have moles, which here are moles divided by liters, so we have to figure out how many liters of solution we have, so how can we convert 500 milliliters to liters?
More Interesting Facts About,
molarity dilution problems solution stoichiometry grams moles liters volume calculations chemistry...
To convert it, it is worth knowing that one liter is equivalent to 1000 milliliters, so to convert it you need to divide it by th000 or you can simply move the decimal three spaces to the left, which is equivalent to dividing it by th000, so 500 m is the equals .5 l, so let's divide the moles by the volume, so now we have the moles above and the moles below and that will give us the marriage of the solution, so 80 divided by 40 is 2 and 2 / .5 , well, if you multiply the top and bottom by two, this will be 4 over 1, so the amount of the solution is 4 moles per liter or simply 4 M.
Now you can convert g to moles and then use the equation to take moles divided into liters, but it is the same. Since what I have here, let's try another problem: how many moles of sodium bromide are in 400 milliliters of an A3 molar solution of NAB? So this time we have marity and volume and we need to find the moles to know that the moles are equal to marity multiplied by the volume but I'm going to set it up as a conversion process marity is moles of a liter so we have 3 moles times a liter of solution, that's what a 3 mole solution means, it's the number of moles per liter and we have 400 milliliters, but we're going to convert that to liters, so we need to divide it by a th to do that, so 400 divided by a th000 is the same as 4 l, so we're going to put it on top and we're going to configure it like that. one way liters cancel out gives us moles so it's 3 * 43 * point 4 if you type that into the calculator you should get 2 so that's how many moles of sodium bromide we have in this solution .
This time we need to find the mass of sodium. chloride given volume and marriage, so how can we do that? In the last example we used marity and volume to get moles, so we only need to use molar mass to go from moles to grams, so the same steps that we have used in the last example, we will use in this example, so let's start with marity, we will write it as 0.5 moles per 1 liter of solution. Now 300 milliliters is the same as 3 L if you divide 300 by 1000. We could see that liters will cancel out and now we have moles of sodium chloride, so we need to find the molar mass of NF, the atomic mass of sodium is 23 and Florine's atomic mass is 19, so when you add that up it gives, let's see 23 + 19 9 and 3 is 12 transfer 1 1 + 2 + 1 is 4 so it's 42 so we have 42 G per mole because we have the unit moles in the top left, we need to put the unit moles on the bottom right so they will cancel out, so now we can multiply everything by 42 * 3 * .5 and that's about 6.3 G of sodium chloride, that's how you can calculate the mass If you are given the volume and marity calculate the volume needed to produce a molar solution of A8. using 37 G of calcium hydroxide, so how can we find a volume in liters or in milliliters if we have the marity and the GRS?
The way you want to set it up is to start with a mass that is G over one and then you want to use the molar mass found on a periodic table to go from G to moles then the grams will cancel out now you can use marity to go from moles to liters because marity is moles over liters you just have to turn it around if you divide by Mari the units will be liters over moles so the moles will cancel out and you will have liters and if you want you can convert that answer to milliliters using the conversion from liters and milliliters so feel free to pause the video if you want to try the problem or you can uh just keep watching so let's start with 37 G of calcium hydroxide so to convert from grams to moles we need the molar mass of calcium, it is about 40, now the hydroxide o o is 16 H is 7, so for the combined hydroxide it is 17, but we have two hydroxides 17 * 2 are 34 and 40 + 34 are 74, so there are 74 G per one mole of substance.
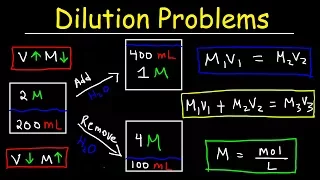
Now let's use marity to convert from moles to liters, so there are 8 moles per 1 liter of solution, so let's see what this answer is. liters so 37/74 that's 0.5 id8 so what we have is 625 L now if we want we can go one step further and convert it completely to liters 1 liter is equal to 1,000 Millers so we can see that the unit GRS cancel moles cancel and liters cancel then it will be 625 * 1,000 so our answer is 625 M if we want it in this unit or 625 l so now you know how to use marity and grams to get the volume needed now before we start this question let's talk about some things so Let's say if we have a solution and the volume of the solution, let's say it is 100 Miller and the concentration is 1 M and let's say the substance is sodium chloride, then what are two forms or what is one form? in which we could increase the concentration and one way we could decrease the concentration of the solution, how can you do it so well?
If you want to increase the concentration, one way to do it is by adding more solute, you can add more sodium chloride. to the solution the other way to increase the concentration is to let the solution evaporate so if water comes out of the solution the volume will decrease therefore the marity will increase so let's say if you don't want to change the amount of solute in a solution needs to decrease the volume of the solution, so if you decrease the volume of the solution by a factor of two, say if it is now 50 milliliters instead of 100, the concentration will double, so which will be 2 m instead of 1 M if you reduce the volume, every time you decrease the volume the marity will increase assuming you have the same amount of solute, if you add more solute the marity will increase and the volume will increase slightly now another way The only way to decrease the concentration without changing the amount of solute in a solution is by adding water, so if you add H2O, the volume of the solution will increase, so if you double the volume, the concentration will decrease by a factor of two. so let's say the volume is now 200 milliliters, the concentration will be 0.5, so if you add water, the concentration decreases, the solution becomes diluted.
If you remove the water by evaporation, the solution becomes more concentrated, so make sure you understand those concepts as they relate to uh. dilution problems now let's work on this particular problem so we have a 300 ml solution of 4 M copper chloride and it is diluted to 900 M which means we are adding water to increase the volume to 900 M so the amount of copper chloride solute that is is not changing in such situation we could use this equation M1 V1 is equal to M2 V2 so before adding water the marity was four i.e. M1 the volume was 300 M now we are looking for the new concentration after adding water and the volume has increased to 900 now let's think about this problem conceptually before using the equation, the volume increased from 300 to 900 so it increases by a factor of three and each time As the volume goes up, the marity will go down, the solution will be dilute it is less concentrated, so if we have a four M solution, it should be reduced by a factor of three, so 4 ID 3 is simply 4/3 or 1, 33.
This should be our answer, so to solve for M2 we need to divide both sides by 900. Now we can get the answer, so we need to type this into the calculator, so 4 * 300/900 is actually 1.33, so that is the concentration of the new solution after adding 600 milliliters of water to increase the volume from 300 to 900. Try this. problem to what volume should you dilute a 200 M solution of 0.5 m lithium chloride to create a solution with a concentration of 10 m then notice that we want to decrease the concentration, therefore we have to increase the volume, so Our goal is to calculate the final volume, so if we reduce the concentration by a factor of five, we will go from 05 to 10, so if the concentration decreases by a factor of five, the volume should increase by a factor of five, so which 200 * 5 is a th000, so that's how you can see the answer conceptually, but let's use the equation to get the same answer, so M1 V1 is equal to M2 V2, so before diluting the marriage is 0.5 and the volume is 200 milliliters in this equation the volume could be milliliters or liters. it just has to match if V1 is in milliliters V2 has to be in milliliters if V1 is in l V2 has to be in liters M2 is 10 and then let's solve for v2 then we have to divide by 10 so if you write it 0.5 * 200 / with 10 you will get th000, so we need 1000 milliliters as the final volume, which is equivalent to one liter.
How many milliliters of water should be added to 100 milliliters of a molar solution of Koh A8 to dilute the concentration to 2 so that we have the marity the volume another marity and we are looking for our volume when you see you know you have to use this equation M1 V1 is equal to M2 V2 so the original solution has a marriage of 8 and the volume is 100 the new solution has a marriage of .2, so first we need to find V2, the final volume, so let's divide it by 2, so 8 * 100/20 you should get 400 MLL as final volume. Now keep in mind that this is not the answer, this is the volume we need.
To get to that now we initially have a 100 milliliter solution, we need to increase the volume to 400 MERS, so how much water should we add? That's the key word here. We want to know how much water to add, so if we have 100. and we need to get to 400, how much more do we need? We are missing 3 100 100 + 300 is 400, so to get to 400 we must add 300 M of water and that is the answer for this problem, so be careful with the questions. where you have to find out how much water you need to add is the difference between the final volume and the initial volume 400 - 100 is 300 consider this problem how many milliliters of water must be removed by evaporation to increase the concentration of a 200 M The solution is also 0, 25 M and we want to increase it to a new concentration of 1 M, so this problem is similar to the previous one, but we need to find the difference between the initial volume and the final volume because we are removing water. the final volume is going to be less this time so let's use the same equation M1 V1 is equal to M2 V2 so before removing the water the marity is 0.25 and the volume is 200 the new marity is one so if the marity increases the volume has to go down so we have to find V2 so we can ignore the 1 * V2 is just V2 so it's actually 0.25 * 200 or 25% of 200 which is 50 so it's V2 so initially We have a solution of o that has a volume of 200 milliliters and by evaporation we want to reduce that volume to approximately 50 milliliters.
How much water should be eliminated? Well, that will be the difference between 200 and 50, which is 150, so we need to take out 150 milliliters ofwater to increase the volume. concentration from 0.25 to 1 now, what about this problem? What volume of 35 M sodium hydroxide solution is required to completely neutralize 82.1 M of 36 M HT solution? So how can we solve this problem? First, you have to recognize the difference between this problem and the ones we've been doing before in the previous examples, we only had one type of solute, it could be sodium chloride Koh, but there was no reaction. We were adding water or removing water.
In this particular problem, we have one reaction, we have two different ones. substances and for these questions you have to be careful now it turns out that you can use the equation M1 V1 is equal to m2v2 but you have to be careful how it is used htl is a monoprotic acid it has one hydrogen per unit formulate sodium hydroxide it has one hydroxide per formula unit because of that, this equation will work as is, you don't have to modify it, but in other examples let's say that if you have a diprotic acid with sodium hydroxide when you balance the reaction, you don't use a one:1 ratio like in the case of this reaction, so you need to modify this equation for those situations that we will go over in this video, but for this particular example, if we were to write a reaction between NaOH and HCL it will be a ratio of one: one uh when NAC is combined.
I mean, when this reacts, the na and CL part will combine to form NaCl. H and O will combine to form water. This is a double response Bas reaction. Notice that it is already balanced. and the ratio M between NaOH and htl is 1, so for this example we can use the equation M1 V1 equals M2 V2, so let's say the left side is for sodium hydroxide and the right side is for HCL, so the marriage of NaOH is 35. When looking up the volume of sodium hydroxide, the quality of the HCL solution is 36 and the volume is 82.1, so since V2 is in milliliters, V1 will be in milliliters, so let's divide both sides times 35, so V1 will be 36 6 * 82.1/35 and you should get 84.4 milliliters, that is the volume of sodium hydroxide that is required to completely neutralize this reaction, this is how you can solve for marity or volume for a acid-base titration problem, just use that equation now let's solve it another way, using a conversion process for the conversion process, I would write the equilibrium reaction even though we know it's one: one, so our goal is to find the liters of sodium hydroxide and then we will get the milliliters, since we are looking for the volume of NaOH, we start with the other substance HCL, so we have 36 moles of HCL per one liter of solution that we must multiply by the volume of the solution.
The volume of the htl solution in liters is 0821 L. Remember to convert from milliliters to liters by dividing by TH or move the decimal point three units to the left so that the liters cancel out and we have moles of HCL, so now we need to convert from HCL to NaOH whenever you want to switch from one substance to another substance that you need. use the mole ratio of the equilibrium reaction, which is 1:one, so for every Mo of HCL that reacts, one Mo of sodium hydroxide will react with it, so the moles of htl will cancel out.
Now our goal is to find the volume and we need to use marity to convert from moles to liters, but we have to divide by marity, so instead of writing moles over liters we need to write liters over moles, so we have a solution of NaOH A35 M, so it is 35 moles of NaOH per liter and we convert liters. again to milliliters, so there are th000 milliliters per liter, so the moles of NaOH cancel out the liters and we should get our answer, so 36 times 821 is 0 29556, take that number divided by 35 and then multiply by th000 and you should get 84.4 milliliters, which is the answer we got using the other method M1 V1 equals m2v2 so now you know how to solve this particular problem both ways.
Now what about this problem? We have a diprotic acid instead of a monoprotic acid. It took 42.5 milliliters of a 25 M K solution to completely titrate 47.1 Mill of a suric acid solution determine the unknown concentration of the h2so4 solution now let's use the equation M1 V1 = M2 V2 is there a way to get the answer without writing the equilibrium reaction so here is K it has one hydroxide per formula unit and here is h204 which has two hydrogen ions per formula unit so M1 V1 will correspond to Koh and M2 V2 will correspond to the acid solution now because we have two hydrogens, put a two in front of M2 V2 since we only have one. hydroxide, put one in front of M1 V1 and it will work.
By doing this, you are incorporating the 1 to 2 mole ratio when balancing this reaction. This is how you can write the equation properly without having to write the reaction, so let's solve it. so the marriage of Koh is 25 the volume is 42.5 and we are looking for M2 V2 is 41.7 so let's solve for M2 let's divide both sides by 2 * 41.7 now there is an error that I need to fix and that error was a number that I plugged in but maybe I already dresses. I didn't understand it until now, so this is what we had before and we said that M1 is 0.25 V1 is 42.5 M2.
We are looking for that now V2, this is where the error I had was. 41.7 but it should be 47.1, it's very easy to change two numbers, so when solving for M2 it will be a little different, so it will be 0.25 times 42.5 divided by two and then we will divide that result by 47, 1 and we should get 3 as marriage. For the solution, I will now show you another way you can write the equation we have using a balanced reaction action. It's a little different from the way we got it, so let's first write down the reaction Koh plus h2so4, so it's a K double replacement reaction will pair with s so4 and as long as the hydrogen pairs with the hydroxide, you'll get water as a product for any acid based titration reaction where you add a strong acid and a strong base you will always get water and a salt, now be careful with the salt formula, it's not just kso4, you have to balance the charges.
K has a charge plus one. Sulfate has a -2 charge, so using this crosslink method it will be K2 so4, so now let's balance the reaction. Notice here we have two hydrogen ions so we need two hydroxides so now there will be a 2:1 ratio for every two hydroxides you have, if you react with two hydrogens you will get two water molecules. Notice that the reaction is already balanced at this point now let me show you how to obtain the equation using the equilibrium reaction coefficients and it is important to understand this method because sometimes you have to use this method instead of the other so let's give this method one name, let's call it coefficient method, the method that Ed in the last example, let's call it subscript method, so for the coefficient method, M1 V1 will still apply to Koh, so I wrote it directly below Koh and, at Same as before, m2v2 goes to correspond to h204, if you do it the other way around you will get the wrong answer.
Now for the coefficient method, coefficient two is going to move to the right, so we're going to put a two in there and coefficient one is going to move. on the left, then for the coefficient method you need to use the criss-cross method to write the numbers in front of M1 V1 and M2 V2 so that you can get the correct answer. Notice that this is the same equation we had in the last method. Now using the subscript method I just want to compare them side by side we need to use the subscripts the two and the invisible one for the hydroxide for the subscript method you don't need to crisscross it so to speak the one just goes here and the two just goes here here and you get the same equation, so for an acid-based titration it is better to use the subscript method because you don't need to write the reaction, but for other titrations, particularly Redux titrations, you can still use this equation, but you want to use the coefficient method because subscripts won't help you.
You don't have hydrogens or hydroxides for the reduxx titration that you'll see in the example below, so you can use either of these two methods you'll get. the same answer, so now let's get the same answer used in a conversion process, so our goal is to find the marriage of h2so4, so we need to get the moles at the top and the moles at the bottom, since we need to find something related to h204 and start with the other one. substance ideally the marriage of Koh, so it will be 0.25 moles of K in 1 liter. Marity is moles of liters.
Now let's multiply by the volume of the K solution. 42.5 is the same as 0425 L. You have to divide 42.5 by th000 to convert milliliters. to liters so liters cancel at this point we need to change the substance from K to h2so4 using the ratio M of the balanced reaction and it is a ratio of one to two and that is why you have to have one and two in front of the M1 V1 Equation M2 V2, you have to incorporate the M relationship to get the correct answer now, for every two moles of K that react, since there are two in front of Koh, one mole of h2so4 reacts with it, so the unit moles of K are They will cancel, so we have. moles of h24 to find the marriage we need to divide the moles by the liters so the volume of suric acid is 47.1 but we need it in liters so it will be 0471 if we divide it by th so now we have moles in the upper liters. at the bottom we now have the amount of the h204 solution so it's 0.25*0425/2 and then take it like this /0471 and you should get 113 M.
This time we have a Redux titration so we don't have any diprotic acids where you can use the subscription method if you are GNA use M1 V1 is equal to m2v2 for this particular problem you have to use the coefficient method so it took 37 milliliters of a 28 molar rum2 chloride solution to fully titrate 61 milliliters of Potassium on page What is the concentration of the km4 solution, so you need to identify the substance of interest, we need Fe2, which is associated with the Fe+ 2 ion, and km4, which is associated with Pagate, so notice that the molar ratio is 5 to 1, so M1 V1 will correspond to fe+ 2. and M2 V2 is going to correspond to pay, so the five are going to move here and one is going to move on this side using the coefficient method, so now let's solve it so that the marriage of Fe is 28 and the volume is 37.
Looking for M2, the marriage associated with the potassium page solution and the volume is 61, so if we divide both sides by 5 * 61, we should get the answer, so M2 will be equal to 28 * 37/5, which is currently 2.72. divide result by 61 and you should get 03396. This is the answer. Now let's solve it using a conversion process because there are some problems where you have to do a conversion, particularly those where you have marity volume and if you have to find GR, then we are looking for the concentration of km4, so let's start with the other substance fe2, so it will be 28 moles of fecl2 per liter of solution and then let's multiply by the volume of F2 in liters, so 37 milliliters is 037 lers if you divide it by th000 then the liters cancel out now we need to change the substance of fe2 to M4 minus, so we need to use the mole ratio which is 5 to one, so for every five moles of fe2 plus, which is the same as fe2, one mole of M4 minus, which is the same as one Mo of km4 that is going to react inside, then these two cancel and to find the concentration we simply need to divide by the liters of the solution. 61 m is the same as 061 l, so we have moles at the top and liters at the bottom. that will give us marriage, so now we just have to write it 28 * 037/5/061 and you will get the same answer 033 967, so that is the marriage of the solution k 4.
Now let's go over some solutions through geometry problems in that we cannot use the equation M1 V1 equals m2v2, so if we look at this question, how many grams of zinc metal is needed to completely react with 79.4 M of a 375 M htl solution? Note that we want to find the GRS of zinc. in that case grams are not part of the equation M1 V1 is equal to m2v2 so we have to use
stoichiometry
so the first thing we need to do is balance the reaction so that we have metallic zinc plus htl what products will we produce in this reaction?Whenever you have a pure element in a compound, it is usually a single replacement reaction. The zinc metal will displace the hydrogen out of the solution and the zinc will pair with the chloride ion if you look at the series of activities if you go to Google. In the pictures you will see that the zinc is above the hydrogen, which means that the zinc is strong enough to displace the hydrogen out of the solution. What is the formula when zinc and chlorine come together? Zinc generally has a charge plus two, chloride generally has a charge minus one, so.
If you combine these two using the cross-link method, it will be zn1, so we don't have to write one cl2, so it will be one of the products and the other product will be Elemental Hydrogen as a pure element. Hydrogen exists as a diatomic molecule, understand the conditions, zinc is a solid, most acids dissolve in water, soaqueous zinc chloride is soluble and hydrogen is a gas, so now we need to balance the reaction, so we have two chlorine atoms on the right side, so we need to put two. vs HCL at this point the reaction is balanced we have two hydrogen atoms on both sides one zinc atom and two chlorine atoms so now we can work out what we are looking for so we need to find the grams of zinc let's get started with the other substance let's start with the amount of HCL, so we have 375 moles of solution, which means there are 375 moles of HCL for every liter of solution.
The volume of HCL is 0794 L. If we divide the 7 9.4 M by the 000, we will obtain. change to liters and then these units will cancel out, so now that we have the moles of HCL, let's convert them to moles of zinc so we can see that the ratio M is 1 to two, so for every two moles of HCL that react , one mole of zinc reacts with it, so at this point we need to find the molar mass of zinc, so we have to go to the periodic table and zinc has a molar mass of 65.3 n, so there are 6539 G of zinc for every mole of zinc, so the units the moles of HCL cancel out and the moles of zinc also cancel out, so this will give us the answer to the problem, so it's 375 times 00794 divided by two times 6539, so the answer is 9735 grams of zinc, so take a minute, pause the video and try.
In this problem, 7.43 G of aluminum is required to completely react with 95.4 M of a copper chloride solution. What is the marriage of solution C2? Again, in this problem we have grams, so we can't use M1 V1 equals M2 V2, so we're looking for the copper chloride junction. Let's start with the other substance, aluminum, but before we do that we need to write a balanced reaction, so we have metallic aluminum and cucl2, so we have a pure element plus a compound, so this is another unique replacement. The aluminum reaction will displace the copper out of solution and the aluminum will pair with the chloride ion.
Now in the activity series aluminum is well above copper, so this reaction will proceed to the right. It's going to work. Aluminum is in group 3A of the periodic table so it has one charge plus three. Chloride is a halogen that generally has a negative charge, so using the cross-link method, when these two come together, they will form a compound known as chloride. of aluminum al3 and the copper will be displaced out of the solution as metallic copper, which is simply CU, so now let's balance the reaction, notice that we have three chlorine atoms on the right but two on the left, which is the least common multiple of two and three one way to find out is to multiply 2 and 3 2 * 3 is 6 so we need six chlorine atoms on both sides, so 6/2 is three, let's put a three in front of the copper chloride 6 ID 3 is 2, let's put a two in front of al3, so notice we have six chlorine atoms on both sides, now we have three copper atoms on the left, so let's put a three in front of Cu and we have two aluminum atoms on the right, so let's put a two in front of an exit, so now the reaction is balanced and now we can answer the question we are looking for about marriage. of copper chloride, so let's start with the other substance, aluminum, so we have 7 43 G of aluminum and let's convert that to moles, so the mass M of aluminum is about 26.98 G per 1 mole, so now we can change the substance using the ratio M, so the molar ratio between aluminum and copper chloride is 2 and three, so for every 2 moles of aluminum that react, three moles of copper chloride react with it, so that these units are cancelled.
Now our goal is to find marriage if you want to find the marriage we are going to. are moles divided by liters, so we need to put liters in the bottom in the next part, so the volume of copper chloride is 95.4, which is 954 L and now we have the number of moles over liters, so that the answer will be 7.43 / 26.98. * 3/2 and then take that result divided by 954, you should get 4.33 M for the purity of copper chloride. Here is another problem: what volume of a 35 mol NAC solution is required to completely react with 39 M lead nitrogen, so let's write the reaction so that we have one compound plus another compound, usually this is a double replacement reaction, so the sodium will pair with the nitrate.
Sodium has a charge plus one. Nitrate has one charge minus one, so as long as the charges are equal, we could simply pair the two. Now, in a one:1 ratio for lead and chlorine, we cannot make lead have a charge plus two and chlorine have a charge minus one, so using the cross-link method it will be pb1 cl2, which we can write as PB cl2. now let's go ahead and balance the reaction so that we have two nitrates on the left side, we need to put a two in front of na3, so we need two in front of NAC and everything else is balanced at this point, now sodium chloride is soluble. of alkali metals such as sodium, lithium and potassium, are always soluble, so we will write that Aquis nitrates are always soluble, however, lead chloride is insoluble.
Chlorides are usually soluble, except with silver, lead, and mercury, so it is a solid whenever two aqueous substances are mixed. solutions and if you get a solid product, this is also known as a precipitation reaction. Now our goal is to find the volume of NaCl, so let's start with the other substance, lead nitrate, so we have 0.15 moles of PB n32 per 1 liter and the volume of the lead nitrate is 39 M or 0039 l , so the liters cancel out. Now we need to change the substance, so we have to use the ratio M, which is 1 to two, so for every mole of pb3 2 that reacts, two moles of NAC react with it and So now these units cancel out.
Our goal is to find the volume so we need to use marity to convert from moles to liters so you have to divide by marity so there are 35 moles of NaCl at the bottom based on this number and one lit at the top . but let's convert it to milliliters there are 1000 milliliters per 1 liter so the moles of NAC cancel out and the moles and liters also cancel out so now we can get the final answer so it will be 0.15 times 039 times 2 /35 and then times 1000. the answer is 33.4 NAC Millers, that is the volume of Na that is required to completely react with all the lead nitrate that is in a solution.
Now that you know how to do the stereometry of the solution, you know how to find the grams, the volume, and the marriage if necessary. 31 m of a 25 molar NAB solution reacts with excess Cl2 gas after the reaction is complete. 481 g of br2 were collected, what is the percentage yield to find the percentage yield? is equal to the actual yield divided by the theoretical yield multiplied by 100% of the actual yield. The yield is usually given in the reaction and is associated with the amount of product that was collected, so the actual yield is 481 G. We have to find a retical yield now, if the actual yield is associated with br2 and is in grams, the theoretical yield must also be associated with grams of br2, so let's use
stoichiometry
to calculate the maximum amount of br2 that can be collected in this reaction .The maximum amount represents the theoretical yield, so we must first write an equilibrium reaction to have chlorine gas reacting with sodium bromide. so we have a pure element and a compound, so this is a simple replacement reaction, in the other simple replacement reactions typically Al, a metal like aluminum would displace another metal like copper out of solution or, in a rare case, a non-metal like hydrogen. from this solution now hydrogen, although it is a non-metal, is similar to metals in the sense that hydrogen forms more one charges metals, they like to form positive charges, most other non-metals like to form negative charges, so chlorine will be replaced by a non-metal. or displace bromine, another non-metal out of solution, both bromine and chlorine like to form negative charges, so when chlorine pushes out bromine, chlorine will pair with sodium, sodium has a plus one charge , chlorine has a charge minus one due to the magnitude of the charges. are equal, these two will come together in a ratio of one:one and the elemental bromine will be displaced out of solution.
Bromine is a red liquid. Chlorine is a gas. And sodium bromide and sodium chloride are here in the solution. they are very soluble whenever you have an alkali metal like lithium, sodium or potassium it is always soluble in water so now we have a balanced reaction we have two chlorine atoms on the left side so we have to put a two in front of the NaCl, we have two bromine atoms. atoms on the right so we need a two in front of nabr and now the reaction is balanced so let's calculate the theoretical yield of bromine in G so chlorine is the excess reactant so we don't have to worry about that, which means sodium bromide is the lemon reagent, so let's start with that, so we have 0.25 moles of nabr per one liter of solution and the volume of nabr is 031 l, so now let's convert the substance of sodium bromide to bromine, so the ratio is 2 to 1, so for each 2 m of sodium bromide reacting with one mole of br2 will be produced, so these units now cancel out.
The last thing we need to do to get a theoretical yield is convert the moles to GS, the molar mass of atomic BR is 79.9, so if we multiply that by two, the mass M of br2 is 159.3max: for bromine it is 619 grams of br2 now it is important to understand what this number means this number means that if all the sodium bromide in a solution reacts, this is the maximum amount of bromine that can be produced in a reaction this is the theoretical yield this is the maximum that can be produced can get from a reaction now we didn't get as much we got less in this experiment we only got 481 so now we can calculate the theoretical yield I mean the percentage yield so the percentage yield will be equal to the actual yield of 481, calculated by the theoretical yield of 619 times 100%, so you should get 77.7%, so that's the percentage yield of this particular reaction of 10 G of aluminum. is placed in a 450 ml solution of a 1.35 mol h2O4 solution, what is the theoretical yield of hydrogen gas produced in grams and how much excess reactant remains?
Now the first thing we need to do is write a balanced reaction so that We have metallic aluminum reacting with sulfuric acid, so we have a pure element mixed with a compound and we know that hydrogen gas will be produced as a pure element and a compound will give a single reaction of replacement, so the aluminum will displace it. hydrogen from the solution and then the aluminum will pair with the sulfate as an ion. We know that aluminum has a charge plus three and sulfate has a charge minus two, so to write the formula when aluminum combines with sulfate it will be al2 so43. whenever you have multiple polyatomic ions you need to enclose them in parentheses so this is what we have now so now we need to balance the reaction.
Notice that we have three sulfates on the right side, which tells us that we need to put a three in front of h2so4 now we have six hydrogen atoms and two on this side, 6id two is three, so we need a three in front of H2 and we have two aluminum atoms on the right, so we need two in front of Al and one here and Now the reaction is balanced, so how can we find the theoretical yield of hydrogen gas? We have the mass of aluminum, since it is a solid and suric acid usually dissolves in water, so it is a solution, then we have the volume and marriage of this solution.
So, we are given the information for both reactants, so whenever you have a situation like this, you need to identify which one is the limited reactant and which one is the excess reactant. Now there are different ways you can do it. One method is the one that can. Find the moles of each reactant and divide it by its respective coefficient, then let's say that if you have two moles of aluminum and two moles of suric acid, for example, for aluminum you would divide it by two, that is the coefficient that corresponds to it, and for sulfuric acid we need to divide it. by the coefficient of three, so 2 over 2 is 1 and 2 over 3 is 67, so in this example, because suric acid has the lowest molar ratio per coefficient, it will be the reaction limit, without However, it will take time to convert. the grams to moles and for sulfuric acid we need to convert the marity and the milliliters to month, that will take time, but you can do it that way if you want another way to do it is you can take the 10 G. of aluminum and convert it to g of gas hydrogen and then take the amount and volume of suric acid and convert it to GRS hydrogen gas separately, whichever gives you the smallest amount of product that is the correct actual yield and at the same Once you know what the reactant is going to be limit, the reactant that gives you the theoretical yieldThe lower the limiting reactant, so by doing it that way you can find the actual yield and identify the limiting reactant and excess at the same time.
I'm going to do it that way, I think it's more efficient, so let's start with 10 G of aluminum. You can try this too, go ahead and convert it to grams of hydrogen, so we need to do a gram to gam conversion. to convert GRS of aluminum to moles of aluminum using mass M and then we need to use ratio m to convert moles of aluminum to moles of hydrogen and then using mass M of hydrogen we can go from moles of hydrogen to gr of hydrogen , then the mass M for aluminum is 26.98 G times one Mo, so the gram of a will cancel out.
Now we need to use the mo ratio between aluminum and hydrogen, so it's a 2:3 ratio, for every three moles of hydrogen. The gas that is produced two moles of aluminum reacts and then these units cancel out and now we need to convert from moles to GRS, the atomic mass of a single hydrogen atom is approximately one, so for a hydrogen molecule that has two atoms of hydrogen, the mass M is two. is 2 G of hydrogen for each mole of the diatomic molecule, so now let's get the answer 10/26.98 * 3/2 and multiplied by 2, so the molar mass, I don't mean the mass M, but grams of hydrogen, it will be 1.111 G.
So, if all 10 G of aluminum react, the maximum amount of hydrogen we can obtain is 1.11. Well, the last number should be two 1.12 G of hydrogen, so I'm going to place this number right under the aluminum so we know that this. are the grams of hydrogen that can be produced if all the aluminum reacts. Now let's start with sulfuric acid, let's convert it to G of H2, so let's start with marity, so there are 1.35 moles of h2so4 per liter and we have .45. L of h204 then these units cancel out and now we need to change the substance we need to go from h204 to H2 so the ratio M is 3:3 so for every three moles of H2 that are produced in a reaction 3 moles of suric acid are consumed in a reaction and our last step is to convert from moles to G and we already know that the mass M is 2 G per mole of H2, so the moles of h204 cancel and the moles of H2 will also cancel, so the answer is is 1.35 multiplied by 45 and 3 over three cancellations, so we could ignore that and then multiplied by two, so we should get 1.25 G of H2, so if all the sufuri acid reacts, the maximum amount of grams of hydrogen that can be produced from it is 1.25 G.
So what is the correct theoretical yield of H2? Is 1.1 1.2? By the time we get 1.1 G of hydrogen, all the aluminum will have reacted, so if there is no more aluminum to react with, the reaction stops, so the correct retical yield is the lowest. of the two values now that we have the correct retical yield, the reactant that gives the correct retical yield of the smallest amount of product which is the limited reactant, so aluminum is the limited reactant, gave us a smaller amount of G of H2 and suricum. the acid is the excess reactant now that we know how we can calculate the amount of excess reactant left, because the excess reactant is a solution we want to find the volume of h2so4 left, how can we do that to understand the To find the amount of excess reaction left, you need to know how much was there before the reaction, that is, the total amount minus the amount that was actually consumed in a reaction or the amount that reacts and that difference will be the amount left. is left over, so for example, let's say that if you have 100 ml of solution and of that only 70 MERS of solution react, then the amount left over is 30 m3, we already have the total volume which is 450 MERS of solution, we need to find out what volume of h2O4 reacts in a solution and then we could figure out how much is left to find out how much h2O4 reacts in a solution, start with the limiting reactant and convert it to the excess reactant, that's what to do so we need to start with grams of aluminum What are they. the 10 G and we need to convert it to the lers of h204 but we will use milliliters for now we will convert it to ml so let's start with 10 G of aluminum now let's convert it to moles using the mass M the mass M is 26.98 times 1 mole so now at this point we need to convert the substance from Al to h204 and we could see that the molar ratio is 2 to 3, so for every two moles of aluminum that react, 3 moles of h204 react with it, so now let's convert to liters , so let's use the marity to make the marity of h2O4 be 1.35, which means there are 1.35 moles per one liter and we can convert to milliliters based on this ratio, there are 1000 milliliters per one liter, so the moles of aluminum cancel out moles of h2so4 also cancel out and liters cancel out so now we can see the volume of h204 that reacts so it is 10/26.98*3/2*1000/1.35 and it should get approximately 41183, so that is the amount of h2so4 that reacts in a solution and now that we have that we can find out how much is left, so we had a total of 450 and 4.83 reactions, so the amount left is approximately 38.7 M.
Now sometimes the question can be how much is left in moles or grams, like whenever you get one of the leftover answers, you can convert it to a different unit, for example, we have the leftover volume, we can use it to convert to the remaining moles or GRS, so If we have the marriage of the solution, which is fairly constant, let's start with 1.35 moles per liter and the volume left in liters is 03817, so if we multiply M * n n, I mean M * V marity by volume, that will give us 1.35 moles * 03817 this is the number of moles left over from the reaction, which is 0515 moles.
Now if you want to find the grams left over, convert the moles left over to GRS, so we need the mass M of h204, we have two hydrogens plus sulfur, which is about 32 plus four oxygen, that's 4*16, so which you get a total of 98, so the molar mass of h204 is 98 G per mole, so if you multiply 98 by 0515, the mass left is about 5.05 G from H to s04, so now you know how to find the amount of excess reactant left for a solution by volume, you know how to get the grams and in moles, you just have to be good with conversions, if you can master the unit conversion process, you can answer. any question you are presented with in a test, here is another problem similar to the last one, feel free to pause the video and see if you can get the answer.
We have silver nitrate ag3 mixed with calcium chloride and here we have a compound. mixed with a compound, so this is a double replacement reaction, so let's go ahead and predict the products and balance the equation so we know that AG will pair with cl AG has a charge plus one CL has a charge minus one, so the magnitude of the charges are the same, so we can simply combine them in a ratio of one:1. Now we need to combine calcium with nitrate. Calcium is a group two alkaline earth metal, so it has a plus two charge.
Nitrate is a polyatomic ion with minus one. we charge so we can see that when these two combine we will get ca32 so now that we have the products of the reaction let's go ahead and balance the reaction so that we have two nitrates on the right side so we need to put two in front of ag3 we have two chlorine atoms on the left so we need two in front of agcl and now the reaction is balanced we have a calcium atom on both sides so now we can find the answer to this problem our goal is to find the amount of grams of agcl that can be produced by the way this is a precipitation reaction agcl is a solid everything else is in the aqueous phase nitrates are always soluble and chlorides are generally soluble except with silver, lead and mercury as well Since we have to find the grams of product, which is basically the yield of Threal, they give us the information from both areas, so we don't know what the limiting reactant is at this point, but just like before, we will take each reactant and do it.
We will find out. How many grams of product can be produced from it? Whatever number gives us the lowest threal yield or the smallest grams of product that will be the retical yield and the reagent that gave us that answer is the limiting reactant, so let's start with silver nitro. let's start with the marriage so we have 0.19 moles of ag3 per liter multiplied by the volume of ag3 which is 025 lers so these units cancel out and now we need to use the ratio M so we can see that it is a ratio of 2:2. between ag3 and agcl, for every two moles of agcl produced, 2 moles of ag3 react, so now we need to find the molar mass of agcl, the mass M of AG is 107.9 plus CL, which is 35.45 , so the mass M is 14335 G times 1 mole so9*025 the 2 over two will cancel out, so we could ignore that time 14335, you should get 681, so I'm going to put this number just below ag3, so which will produce 681 G of agcl, so now let's start with the other substance, calcium. chloride, so we have 0.15 moles of ca2 per liter per 031 L and the ratio M between agcl and CA2 is 1 to two, so for each mole of calcium chloride consumed in a reaction, two are produced moles of H and the molar mass is the same, it is 143.5, so these units cancel out and the same goes for that, so it is 0.15 * 031 * 2 times 14335, so chloride of calcium can produce up to 1,333 G of agcl, so clearly that is not the theoretical yield, the maximum amount of htl that we can obtain in this reaction is the lower of the two values, so it will be 681 G, which It means that ag3 is the limiting reactant because it gives us the smallest radical yield and calcium chloride is the excess reactant.
Now let's calculate the number of grams of excess reactant left, but first let's find the volume, so the excess reactant is calcium chloride, the total volume of calcium chloride before the reaction is 31, let's find a volume of calcium chloride that actually participates or that is actually consumed in a reaction, then Start from the limited reactant and convert it to the excess reactor, so let's start with ag3, which is 0.19 moles per liter, and multiply by the volume, which is 25 L , and the ratio M between the limiting reactant and the excess reactant is 2: one. therefore you always need the ratio M when you want to convert from one substance to another, so for every two moles of ag3 consumed in a reaction, one mole of calcium chloride will also be consumed, so these units are cancel and the liters also cancel.
Now our goal is to find the volume, so to go from moles to liters we need to use the marity and that is the marity of calcium chloride, which is 0.15, so that's 0.15 moles of calcium chloride times 1 liter of solution and convert liters to milliliters. so there are 1,000 milliliters per liter, so these units cancel liters cancel and then we have 9 * 025 / 2 take that result by dividing it by 5 and then multiply it by th000, so you should have 1583 milliliters of calcium chloride, that is the amount that is consumed in a reaction, so now let's find out how much is left to have a total of 31 milliliters minus 15.83%.
Let's multiply 15 moles per liter by the remaining volume in liters, which is 01517, which is basically this number in liters and then convert the moles to grams. we need the mass M of calcium chloride, calcium is about 40 CL and it is 35.45 but you need to multiply it by two so you should get 110. n g per mole So it is 15 * 01517 * 1109 and the grams of reactant in The remaining excess is 252 G of calcium chloride. 200 M of a 5 molar sodium chloride solution is mixed with 600 M of a 1 M sodium chloride solution. What is the color of the mixture? So here we have the same substance, so no reaction occurs, we don't have to. write a single or double replacement reaction, but how can we find the purity of the combined mixture?
Let's say if the first solution has 2 moles and if the second solution has three moles, then the combined solution must have five moles of sodium chloride. Note the moles are equal to M1 V1 or marriage time volume, so M1 V1, which represents the moles of sodium chloride in the first solution, plus M2 V2, which represents the moles of sodium chloride in the second solution, they must be equal to M3 V3, which represents the total moles of sodium. chloride in the combined solution, if you add two and three moles, you should get five, so we are using the dilution equation but in its expanded form, this is how you can apply it for mixture problems, so let's find what the answer is, but before we do that before we calculate it, let's see if we can calculate the answer if you mix a concentrated solution with a dilute solution, the mixture has to be somewhere in between, so it has to be between 5 and 1, it has to be greater than 1 but less than five between 1 and 5 the midpoint is three now, do you think the answer is between 1 and 3 or 3 to 5?
Notice that we have more dilute solution if we had the same amount of five M solution and one M solution. The mixture should be right in the middle of the three, this problem is basically a weighted average, but sincewe have more solution than one M, the mixture or the weighted average should be between 1 and 3, it could be like two, it could be 2.5 1.8, but it is somewhere between 1 and three, so let's calculate the answer using the equation, so the first solution has a marriage of five and a volume of 200, the second solution has a marriage of one but a volume of 600 and we are looking for marriage. from the third solution now what is V3 V3 is the total volume if you mix 200 milliliters with 600 milliliters the solution will now contain 800 M note that we can simplify the calculation if we divide each term by 100 so that we can cancel out the zeros 200 ID 100 is 2 and 5 * 2 is 10 600 / 100 is 6 so this is simply 6 and 800 divides 100 is simply 8 so we have M3 * 8 10 + 6 is 16 and if we divide both sides by 8 16 ID 8 is 2 then the marriage of the new solution is 2 m, it is between 1 and 5 but it is less than three because we have more diluted solution. 700 M of a 2 M NAC solution is mixed with 600 M of a three M sodium sulfate solution and 500 M of a four molar sodium phosphate solution.
What is the purity of the sodium ion in the combined reaction? ? If we mix these three substances, they will not react with each other and sodium is a specific ion, so it will not be consumed in a reaction, so to find the marriage of the sodium ion we simply need to find the total moles of the sodium ion divided by the total of liters, we are going to solve it using two methods, the second method would be to use the M1 V1 equations, so find the moles of necl in each solution, so for the first one for NaCl I meant that we want to find the moles of na+ in each solution for the first we have 2 moles per liter multiplied by 7 L, which is 700 m, so 2 * 7 that is approximately 1.4 moles of na+ notice that we have a na in that formula unit now for the second it is a little different we have three moles the marity is three so we have three moles of sodium sulfate per liter of solution and the volume is 600 Mill, which is 6 l, let's cancel, but notice that there are two sodium ions in that formula unit, so there are two m M of na+ for every Mo of sodium sulfate, so these units cancel out and therefore it will be 3 * 6, but multiplied by two. there are 3.6 moles of na+ for the third solution we have a marriage of four so these four m of na3 po4 per liter multiplied by the volume of 5 l then the liters cancel and there are three moles of na+ since the subscript is three there are three moles of na+ times one Mo of sodium phosphate, so this will be 4 * 0.5 * 3, which gives us 6 moles of na+, so now we can find the marriage of ne+, which I'm going to put in parentheses so that the concentration of the sodium ion is the total moles is 1.4 + 3.6, which gives 5 plus 6, that is, 11, so we have a total of 11 moles of sodium ions divided by the total volume of the solution, which is 700 + 600 + 500 700 + 600 is 1300 + 500, i.e. 1,800 1,800 milliliters if you divided by th000 is 1.8 L to find a marriage, the volume has to be in liters, not milliliters, so 11 / 1.8 the marriage is 6.1 M na+ now you may be wondering, is this number greater than four and the reason why is because there's a three in front, so in that four molar sodium phosphate solution you have to realize that there are 12, that's 12 molar na+ if you do four times three, so for this the concentration of na+ in this solution it is 2, for this one it is 3 * 2 it is 6 and for this it is 4 * 3 which is 12 and therefore the concentration of na+ is between the lowest number and the highest number between 2 and 12 which is six , which makes sense now, this is another way we can get the The same answer now, in the last example we were mixing two solutions, so we had M1 V1 and M2 V2 on the left side of the equation and M3 V3 represents the combined mixture.
This time we are mixing three solutions so we should have three things on the left side of the equation so it will be M1 V1 plus M2 V2 plus M3 V3 that is for the three solutions and then M4 V4 for the combined mixture now you have sense because this represents the moles of Na+ in the first solution. are the moles for the second solution and ask the moles for the third solution in the last example or the other method that we used, we added the moles in each solution and divided them by the total volume which is V4 and that gave us M4, so this equation simply helps you do the same job, but it appears to be easier, but the job remains the same.
Now you need to incorporate the coefficient, not the coefficient, but the subscript of Na into this equation, so for sodium chloride the subscript is one. so let's put a one in front of M1 V1. Now for sodium sulfate the subscript is two because there are two sodium ions there so we have to put a two in front of m2v2 and for sodium phosphate we have a three in front so let's put the three in front. If you do that you will get the correct answer so now let's find the concentration of na+ so that the marriage of the first solution is two and the volume is 700 and then plus 2 * the marriage of the second solution which is three and the volume is 600 and then plus 3 * the marriage of the third solution which is 4 * the volume of 500 and we are looking for M4 the total volume 7 00 + 600 + 500 we said it is 1,800 Mill now in this equation we do not need to change it to liters because V1 V2 V3 is already in milliliters, so V4 has to be in milliliters and it has to match, so let's put 1.00 on this side to simplify the math as before, let's divide each term by 100, so 700 ID 100 is 7 7 the zeros cancel out and what is left is 2 * 17 which is 14 600/100 is 6 so 2 * 3 is 6 * the other 6 which is 36 500 divided 100 is 5 5 * 4 is 20 20 * 3 is 60 and 1,800 divided 100 is 18 14 and 36 is 50 50 + 60 is 110 so it is 110 over 18 which is equal to M4 and you are I will get the same answer: 6.1 m, which is the concentration of the ion na+, so that's it for this video, thanks for watching and have a great day.
If you have any copyright issue, please Contact