IV Fluid Types & Uses Nursing IV Therapy: Isotonic, Hypertonic, Hypotonic Solutions Tonicity NCLEX
Jun 19, 2024Hi everyone, I'm Sarah, RN.com RN and in this video I'm going to talk about osmosis of body
fluid
compartments and the differenttypes
of IVfluid
s, so let's get started. Intravenous fluids, also known as intravenous fluids, are special fluids that we administer. to the intravascular space which is part of the extracellular compartment space and the administration of intravenous fluids is a very common treatment that patients receive every time they come to the hospital and as a nurse our role revolves around administering these fluids to this space and we do it based on the healthcare provider's orders and these fluids can be used to treat a wide variety of conditions, really anything that affects these compartmental spaces where we need to replenish that fluid, let's say the patient is dehydrated, it is possible If you need some fluids to help you with that or you are having electrolyte imbalance or acid-base imbalance, we can use these fluids to help correct that, so as a nurse, you want to be familiar with the differenttypes
of IV fluids, such asisotonic
,hypotonic
andhypertonic
, and you also want to know how they work once we administer them. to the body and what you need to monitor while your patient is receiving these fluids, but before we go over these different types of fluids, I first want to go back and review the different body fluid compartments, now that the average adult body is made up of about 60 to 70 percent. percent water, so there is a lot of fluid inside our body and this fluid has to be stored somewhere and there are two main compartments that store this fluid.I want you to remember that the first compartment is known as the intracellular compartment and this is the fluid that is inside the cell and inside the cell, so remember that this is a fluid inside our cell, as you can see here, then we have the extracellular compartment and this is the fluid that is outside that cell, an additional medium Beyond or outside. so we're talking about that fluid that surrounds the cell and it's made up of the intravascular fluid that you can see here, it's also known as plasma, then we have the interstitial fluid that you can see here in blue and it's just hanging around our cells and then we have the transcellular fluid, so now let's take a closer look at these body fluid compartments, the first being the intracellular space, so again this was the fluid found inside the cell and this space actually represents two thirds of the water of our body, so most of our fluid is inside our cells and then there is the extracellular space which again is that fluid outside the cell and it represents a third of the water in our body and includes the fluid compartments like the interstitial fluid compartment and the interstitial fluid compartment is the fluid that surrounds the outside of our cells and this fluid plays a very vital role in helping to be a medium for electrolytes and other substances to move to and from the cell to the plasma with the aid. of the capillaries and the intravascular fluid compartment, which is again known as plasma, is the fluid found inside the blood vessels that contains so many important substances such as electrolytes, blood cells, etc. and lastly, we have the transcellular fluid compartment and this is actually the smallest compartment and this is the fluid that is found within certain body cavities such as spinal fluid, the fluid that surrounds our heart, lungs and joints.

More Interesting Facts About,
iv fluid types uses nursing iv therapy isotonic hypertonic hypotonic solutions tonicity nclex...
Now it is important to note that all of these compartments are actually interconnected with their own amount of water and electrolytes. and they will work together to help maintain a homeostatic environment in our body and the way they do that is they will change the water electrolytes and other nutrients so that we can maintain that balanced environment and they do this by changing through various processes in the body with one of those processes is osmosis, so in healthcare we can administer fluids intravenously, let's say to this intravascular compartment to help expand it if necessary, or shift fluids around these compartments through this osmosis process. to help us correct fluid imbalances or other problems that may occur within the intracellular and extracellular spaces, so to help us understand how intravenous fluids do this, let's talk about osmosis, so osmosis is a process in which the Water will move from a fluid with a higher water concentration to a fluid with a lower concentration, in other words. water will move from a fluid that has a low concentration of solute to a fluid that has a higher concentration of solute and it does it passively, it doesn't need energy or anything from the cell, it actually does it on its own and it does it. through a semipermeable membrane that is only permeable to water molecules, so we will illustrate this process by looking at this drawing.
Here we have our semipermeable membrane that is only permeable to water and on one side of the membrane we have many water molecules but we do not have many solutes and on the other side of the membrane we do not have many water molecules but we do have many solutes, so, according to osmosis, what will happen is that water will move from a higher concentration of water to a lower concentration of water or you can look at it this way, water will move from the place where there are not many solutes to a place where there are many solutes.
Now the big takeaway I want you to know What you get from osmosis is that this process is greatly influenced by the concentration of the solute in the fluid and depending on how concentrated the solute is in that fluid will determine how osmosis It will affect the way water will move from this extracellular space to the intracellular space or vice versa. vice versa, so what is a solute? A solute is a solid that has dissolved in a liquid and there are many different substances that can become a solute in a liquid solution, one of which is like sodium and chloride, so we can take sodium and chloride in their solid form we put them in a liquid every time we do it, once they dissolve they become an electrolyte but they are still a solute in that liquid that we have now, we can take it and we can administer it to the patient in their intervascular system now depending The amount of sodium and chloride that we actually put into that liquid will determine how the osmosis process in this extracellular and intracellular compartment will be affected, which brings me to osmolarity.
What is osmolarity? Osmolarity is the ratio of solutes within a specific volume of liquid. In other words, it is the total concentration of solute per liter of solution, so depending on the osmolarity of the intravenous fluid will depend on how well osmosis will work within the body to move the fluid around these compartments, so we can consider that liquids have a high osmolarity or a low osmolarity, so whenever a fluid has a high osmolarity, we say that it has many solutes in that fluid, whenever something has many solutes, it has less water, on the other hand, if a fluid has a low osmolarity, it has a low amount of solutes means it will have more water and in healthcare we can use osmolarity to our advantage to help treat patients who are sick and need fluid replacement depending on the compartment we need try and change the liquids around us.
Do this by administering various types of fluids that have different osmolarities or solute concentrations that will move water into or out of these compartments. Now let's talk about the different types of IV fluids and their
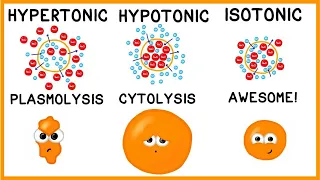
tonicity
, so again there are three main types of IV fluids which areisotonic
,hypotonic
andhypertonic
and let the prefix and suffix of these fluid names help you determine which type of fluid we are dealing with, so the prefix ISO means equal and this means that the solute concentration of the fluid matches or is equal to that of blood. hypo plasma means low and this means that the concentration of solute in the liquid is less than that of the blood plasma, while hyper means high and this means that the concentration of solute in the liquid is more than that of the blood plasma and then when we look at the tonic suffix, this is fortonicity
and it tells us about the capacity of the fluid that will enter that extracellular space, therefore, that intravascular space, how well it will move water in and out of that intracellular space, therefore , inside the cell, or not, it's going to keep it the same or it's going to accumulate both parts and this is based on the concentration of electrolytes, therefore, those solutes in the intravenous fluid, so first are the isotonic IVsolutions
.These have the same osmolarity as blood, so the same concentration of solutes and with these liquids there will be an equal transfer of water, so our cell will remain the same, therefore, we can use these fluids to expand the volume of liquid extracellular, hence our plasma. Now, why would we want to expand the extracellular fluid well if the patient is experiencing fluid loss in this space, such as through vomiting, diarrhea? They need to get some sodium and chloride back or they're experiencing hypovolemic shock Burns or maybe they're going to have surgery and we know that with surgery they're going to lose a lot of blood, like that extracellular fluid, now some fluids that are considered isotonic the normal saline of ringer lactate, also called LR and 5 dextrose in water.
Now there's an asterisk next to this and I'll tell you about this liquid here in a second because although it is isotonic, it works as a hypotonic solution once administered and Some things to remember about isotonic
solutions
is that with normal saline it replaces only water, sodium and chloride and it is the only solution we use to administer with blood, so you do not use any other solution, just normal saline and with this type of solution you have to be careful with fluid overload, especially in patients with kidney and heart failure, because their heart and kidneys don't work very well and if we put too much fluid in them, they can become overwhelmed and we can actually give back too much fluid. in the extracellular space, so you want to monitor your blood pressure, make sure you're not hypertensive, you also want to check your breathing and check those lung sounds, make sure you don't hear any crackles that might indicate pulmonary edema where we have. had fluid buildup in the lungs and then also look at their extremities, especially the lower extremities, make sure there's no edema present and because we're giving sodium and chloride, you want to check those sodium and chloride levels as well, make sure there's no are increasing because This liquid could cause that if we give too much, the next is five percent dextrose in water D5W.This solution replaces water and glucose, so as I noted above, it starts out as isotonic but ends up as a hypotonic solution and why is that okay in this one? The solution is dextrose and when the dextrose enters the body the body will use it therefore it will be metabolized but what is left is not very concentrated it is actually just free water so we have a low osmolarity and therefore Therefore, it becomes hypotonic due to the components of this fluid. it is not for a fluid resuscitation situation, it can actually increase blood glucose causing hyperglycemia, but it can help with hypernatremia, that is when we have too much sodium in the blood and what this fluid will do because it is ultimately hypotonic , a feeling of thinning that blood can help, therefore lowering our sodium level there and then lastly with LR, this solution contains water, potassium, sodium chloride, calcium and lactate.
Now this liquid contains lactate and lactate can actually help increase blood pH by converting it to bicarbonate, which is really helpful always. We have acidotic conditions such as mild cases of metabolic acidosis, however, it is not for patients with liver disease because our liver converts lactate into bicarbonate or for patients who experience lactic acidosis because there is already a large amount of lactic acid in the body, so I don't want to add more and because this liquid contains potassium, you'll want to monitor for hyperkalemia, high potassium in the blood, especially if your patient has any type of kidney failure.
The following are the hypertonic intravenous solutions and these solutions have a higher osmolarity than blood so they have a higher concentration of solutes in the fluid now because this osmosis will cause water to leave the intracellular space therefore it can shrink the cell, which can help expand extracellular space. Why would this be beneficial if we have a patient who is very low in sodium, so they have severe hyponatremia where they don't have much sodium in their blood or they havebrain inflammation, which is cerebral edema. Now some solutions that are considered hypertonic include three percent saline dextrose. 10 percent in water, dextrose, five percent in normal saline and then 5 percent dextrose in half normal saline.
Now, some things to remember about these liquids every time you give them is that you have to use them with great caution because they run the risk of overloading that extracellular space because we're pulling fluid from inside the cell because remember a lot of fluid is remains inside that cell in that extracellular space, which in the end could overload our system and cause pulmonary edema, in addition, because these solutions are highly concentrated, let's say that, like saline, they could cause hypernatremia, so we would like to verify sodium levels in the blood and when you are going to administer these solutions, you will always want to consult your facility's protocol on how to properly administer hypertonic solutions because some may recommend that all hypertonic solutions should be administered through a central line instead of a peripheral intravenous line.
While others may say that only concentrations of 10 percent or more should be administered through a central line and the reason for this is that if hypertonic solutions get into the surrounding tissue, they can be very harmful to those cells and tissues, so we must be careful with extravasation and lastly hypotonic intravenous solutions, so these solutions have a lower osmolarity than blood. therefore a lower concentration of solutes in the fluid and due to this osmosis It will cause water to move from the extracellular space to the intracellular space, which can swell the cell to the point where it can rupture.
Now these fluids will help dilute the extracellular space. and replenish the interior of the cell and why this would be beneficial if there is too much solute concentration in the blood, for example, the patient has hypernatremia. We can dilute it with these Plus Solutions can help provide free water to help the kidneys excrete waste and prevent dehydration in some hypotonic solutions include half normal saline, 0.225 percent saline, 0.33 percent saline and of course five percent dextrose and water, but again, that started out as isotonic, but once in the body it becomes hypotonic, so it swings between both and some things you want.
What a nurse should remember about hypotonic solutions is that they cause the cell to swell because fluid leaves the extracellular fluid and enters the intracellular fluid, which could cause swelling of the brain, so it is necessary to monitor changes in the mental status, low blood pressure, hypovolemia and because we are giving a little free water to the extracellular space, we could give too much, which could really reduce the sodium level, so you need to monitor for hyponatremia and then monitor the patients who are more sensitive to fluids, especially if they have heart failure and kidney failure because they can't handle the extra water, so that concludes this video.
If you want to watch more videos in this series, you can access the link in the YouTube description below.
If you have any copyright issue, please Contact