GCSE Chemistry - The Mole (Higher Tier) #25
Apr 25, 2024In this video we are going to cover what the term
mole
means and see how we can use this formula to convert betweenmole
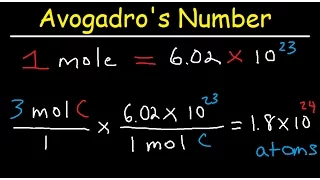
s of mass and relative mass of formula. Now the term mole is a little strange, but it's actually just a unit that we use to measure how much chemical substance we have, just as we measure distance in meters and time in seconds, we can measure how much substance we have in moles and a mole of any substance is just the amount of that substance that contains 6.02 times 10 to the 23rd power. particles with particles referring to atoms, molecules, ions, or even electrons, depending on what substance you're talking about, so If we had a small pile of carbon and were told that it contained exactly one mole of carbon, then there must be 6.02 times 10 to the 23rd power of carbon atoms in that pile we call this number Avogadro's constant and the reason why is This specific number is that the mass of that number of particles of any substance will be exactly the same number as the relative atomic mass or formula of that substance in grams, so our little one-mole stack of carbon would weigh exactly 12 grams because the relative atomic mass of carbon is 12.Meanwhile, one mole of oxygen that has a relative formula mass of 16 times 2, so 32 would weigh 32 grams or 1 mole of co2 with a relative formula mass. of 12 plus 16 plus 16 and then 44 would weigh 44 grams but in all these cases there would be 6.02 times 10 to the 23 atoms or molecules due to this rule we can create a formula that tells us that the number of moles in a sample is equal to the mass of that element or compound divided by its mr so if we want to know how many moles there were in 42.5 grams of ammonia we would do 42.5 which is the mass divided by 14 plus 3 times 1. then 17 which is the mr of ammonia and this would give us 2.5 so we know there are two and a half moles of ammonia in the 42.5 grams we can also rearrange the formula to find the mass if we were given the number of moles, for example what is the mass? of 3 moles of carbon dioxide, this time we would multiply our 3 moles by the mr of co2, which is 12 plus 2 times 16, so 44, which gives us 132 grams of co2, we can also calculate the mass of an element particularly within a larger compound like the mass of carbon in three moles of carbon dioxide for this, all we do is take the number of moles, which is three, and multiply it by the m of carbon, which is twelve, so 3 times 12, which gives us 36 grams of carbon. in our 132 grams of carbon dioxide and if you wanted to go a step further you could subtract those 36 grams from the original 132 grams to find that there must be 96 grams of oxygen because we can see that co2 is only made up of carbon and oxygen now, The last thing we should mention is that when you look at a chemical equation you can think in terms of moles, so for this equation we can think of one mole of magnesium reacting with two moles of hydrochloric acid to form one mole. of magnesium chloride and one mole of hydrogen gas and you should think of this as proportions, so if we start with two moles of magnesium we would have to react with four moles of hydrochloric acid and we would produce two moles of magnesium chloride and hydrogen. gasoline anyway, that's all for today, so I hope you found it useful and I'll see you next time.
If you have any copyright issue, please Contact