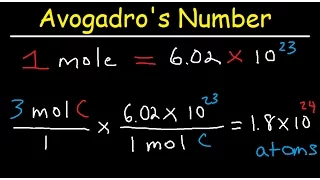
Avogadro's law Practice Problems
Jun 05, 2021In this video we are going to review something known as Avogadro's law. So what do you think is the main idea behind Avogadro's law in relation to the gas laws? Avogadro's law describes the relationship between moles and volume. So what will happen to a balloon? of a balloon, if you blow more moles of gas or air into the balloon, as you increase the number of moles of air in a balloon, the balloon will expand, so as you increase the number of moles represented by the letter n, the volume will increase further. The more air you blow into the balloon, the bigger it will get and moles are basically quantities, think about a dozen, a dozen represents 12, so a mole is 6 times 10 to the power of 23, it's a very large number.
Now let's say that if you have a balloon that contains 1 mole of air it will have a volume of 20 2.4 liters. Now let's say that if you have 2 moles of gas, the volume will increase to forty-four point eight liters, so as you increase the number of moles of gas, the volume will increase. The equation you need to know that goes with Avogadro's law is this equation V 1 over N 1 is equal to V 2 over n 2, so that's the formula you'll use when solving gas law
problems
dealing with moles. and volume now. V 1 and V 2 can be in milliliters or leaders, but they simply have to match, so if V 1 is in milliliters, then V 2 should also be in milliliters.Now the graph that goes with Avogadro's law if you plot y on the x axis. at the end of the y-axis is a direct relationship, so it's just a straight line. Now let's solve some
problems
if 2.4 moles of gas occupy a volume of 60 liters at a certain temperature, what volume will 3.7 moles of gas occupy? So let's write what we know that n 1 is 2 point 4 moles and the volume that corresponds to that which is V 1 is 60 liters and 2 is 3 point 7 moles our objective is to calculate V 2 so let's use the formula V 1 over N 1 is equal a V 2 divided by n 2 so V 1 is 60 + 1 is 2.4 V 2 is what we are looking for and n 2 is 3.7 moles so what we need to do is cross multiply so let's multiply 60 by 3.7 so that's going to be 222 and that's equal to 2 point 4 times V 2 so now we have to divide both sides by 2 point 4 so V 2 is 2 22 divided by 2 point 4 and that's going to be 90 2.5 liters and that is the answer then notice that we increase the moles from two point four to three point seven and the volume increases from sixty to ninety two point five now let's move on to the number two a 250 milliliter balloon contains point three five moles of gas n2 yes point forty and five Moles of gas n2 were added to it, what will be the new volume of the balloon now as before, let's write what we know, okay, let's rewrite that and one will be 0.35 moles and v1 is 250 milliliters, now what is y.. . and what is v2 our goal is to calculate the new volume of the balloon, so we need to find the value of v2 now, since v1 is in milliliters, v2 will also be in milliliters, now what comes into this problem and is not the point 45 moles if Put that there, you won't get the right answer.Notice that the point 45 moles were added to it, so we need to take the point 3 5 and add the point 4 5 to it, which will give us the point 8, so it will be n, its point 80. moles so now we can use this formula and let's substitute what we have so that v1 is 250 n1 is 0.35 let's calculate V 2 and n 2 is 20 80 moles, so let's multiply 250 by the point 80 is 200 and the units we have are milliliters per moles and that is equal to 0, 35 moles times v2 so now let's divide both sides by 0.3 5 moles on the right we could cancel those two values on the left the unit moles will cancel out therefore v2 will be in milliliters so now take 200 and let's divide it by 0.35, so v2 is equal to five hundred seventy-one point four milliliters.
Does this answer make sense if we increase the number of moles from 0.35 to 0.8 and then it makes sense for the volume to increase from 250 to 570 1.4 and that makes sense as the number of moles increases the volume should increase to SHINee, so if you double the number of moles, the volume should double, if you triple the number of moles, the volume should also triple, it's a direct result. linear relationship are directly proportional number three an eighty-five liter flexible container contains 3.4 moles of gas how many moles of gas must be removed to decrease the volume of the container to forty liters now let's write down what we know each time this keeps happening n1 It is three point four moles and v1 is eighty-five liters.
Now we need to find n2 to answer the question and this time we are given v2. v2 is 40 liters, so let's solve this problem using the same formula as we did. We've used before, so v1 is 85 n1 is 3.4 v2 is 40 and our goal is to calculate y 2, so let's start by multiplying 40 by 3.4 and that's 136 liters times moles and that's equal to 85 liters times n2 , so now let's divide both. sides times 85 liters so n 2 will be 136 divided by 85 and that is approximately 1.6 moles as we can see that the unit of liters cancels out and we are left with moles so this is the final answer is n 2 , well, n 2 is 1 point 6 moles. but does that answer the question: how many moles of gas must be removed to decrease the volume, so we start with 3.4 moles and end with 1.6 moles, so how many moles of gas were removed to go from a value initial value of 3.4 to a final value of 1.6, so what we need to do in this problem is calculate the change in moles, so that is the final quantity minus the initial quantity, the final quantity was 1.6 , the initial amount was 3.4, so one point six minus three points. four will give us a negative change value, so one point eight is negative because we decreased the number of moles, that's why it's negative, so one point eight moles of gas were removed from the container and that's the answer, so That's all for this video, thanks for watching.
If you have any copyright issue, please Contact