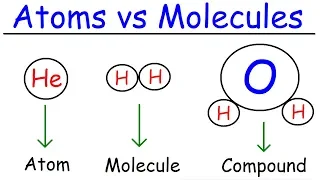
What's the Difference between an Atom and a Molecule?
Feb 22, 2020What is the
difference
between anatom
and amolecule
? If you're wondering this, you're definitely not alone because it's a very common question in chemistry, so the short answer is thatatom
s are like Legos or blocks or bricks andmolecule
s are like these things that we actually build with those blocks of bricks, that's a short answer, let me give you a longer answer that might be more helpful, so let's start by talking a little about atoms, there are some important parts of which an atom has a nucleus that is made up of. of protons and neutrons that'swhat
I've drawn here with little dots and then around the nucleus of the atom which is where the electrons live and the electrons live in these circular neighborhoods called energy levels and that's why I'm drawing the electrons here with these black points, many atoms have more than one energy level, so they have these rings around the nucleus, so with these electrons, well, here's an atom, let me draw another one, here's a proton, here's a neutron, this Anjelica It has a proton and then around the nucleus. here is the electron, here is an energy level that an electron lives in, so there are two atoms here, so there are all kinds of different types of atoms and the type of atom you have depends on the number of protons in the nucleus, This atom here has eight. protons in the nucleus which means it is an oxygen atom, this one here only has one nucleus proton which means it is a hydrogen atom, like I said there are different types of atoms and there are actually about 100 different types and when we talk about them it is usually easier than instead of writing the name each time we use abbreviations, so we want to talk about oxygen we write an O for hydrogen there is an H we have carbon atoms represented by a see we have nitrogen atoms represented by a chlorine N with a fluorine CL with an F and so on all these ABS all these types of atoms are all a little bit different.I like to think of these as Lego blocks, there are all these different ones right there. types of Lego blocks, but they are all a little bit different, there are different colors, they are different sizes, some are tall, some are flat and thin and that's like these atoms that have slightly different characteristics now, the different characteristics of these Lego . Blocks are useful when we really want to build something with them and then we're happy to find out that one is very thin or the other is red or
what
ever, and that's where the different qualities of atoms come into play.We take these bricks and we build like a little house and now we are talking about molecules so we take these bricks and put them together we can do the same with the atoms. I can take an oxygen atom and put it together with two hydrogen atoms and when we say put it together, I mean, I physically connect it, that's what these little lines represent here and when I do that, when I have an oxygen connected to two hydrogens, I have a water molecule, so I call it h2o because there are two hydrogens and one oxygen. or I can do this.
I can take a carbon atom and attach three hydrogens to it, attach another carbon to two hydrogens and then an oxygen and then a hydrogen and I have a molecule called ethanol, which is a type of alcohol found in beer and wine. The point is that molecules are formed when we take individual atoms and physically combine them to make things in the same way we take individual Lego blocks and put them together to build houses or whatever place you want to make, and I'll even take this step. To do this, I show at the beginning that if we could zoom in millions and millions of times, this is what Nana would see.
If we took this water molecule and we could zoom in millions and millions of times, this is what we would see. and we would see the oxygen atom and the two hydrogen atoms and that in reality they are all physically connected to each other, they are physically connected because they share electrons forming what is called a covalent bond, you don't know that yet, don't worry. about that, but they're connected here because they share electrons and now they've made a physical connection with each other and they've let you know something that's like one of these Lego houses where everything comes together, so anyway, that's the
difference
between atoms and molecules, atoms are like individual building blocks and molecules are what we get when we take these building blocks and combine them, connect them together.If you have any copyright issue, please Contact