4.1a Nomenclature of Alkanes
Mar 18, 2024Alright, let's jump into this chapter on
alkanes
by first starting with thenomenclature
ofalkanes
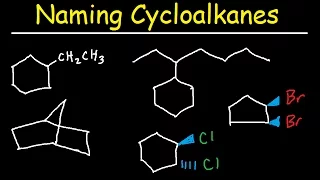
and first of all, an alkane is a hydrocarbon, so only carbon hydrogen generally does not contain carbon-carbon double bonds or carbon triple bonds. -carbon. carbon-carbon single bonds which are our alkanes here and in this case, when we name alkanes, we always name them with a suffix A and E, so here methane, ethane, propane, etc., etc., and then the prefix we put in front depends on the number. of carbons in the chain and in this case you have 12 prefixes to memorize. I have them diagrammed here on the right, so meth f probe up pent hex HEPT OCH non deck on deck doe deck so and if we look at these with five enclosed, so students usually struggle with that once it's a pentagon has five hexagon sides since a hexagon has six sides or ox and said octagon has eight sides or mallet since a decade is ten years students have no problem with those, so a couple doesn't since it sounds like nine students don't bother with and then unzip and exit the platform, some classes don't even learn this, but it's like decade plus one and decade plus two, so ten plus one ten plus two eleven and twelve carbons, respectively, students have no difficulties there, but the rest here, so hepps means seven, which is probably new to you, so the students work with that one that just came to MIT, it's kind of memorized here, but the first four, so math f p-- end of rope, so there's four in a row, they're probably pretty new d a-- and students often, especially at the beginning of the semester, have a hard time with these four, so I'll give you a little mnemonic here, so in that mnemonic I'm. eat peanut butter to get meth, so I eat peanut butter, I know it's stupid, I know it's stupid and that means you're more likely to remember it, but take those four, in fact, take all 12, obviously , because you're going to need to know them, so our first 12 alkanes are for our methane, ethane, propane, butane, pentane, hexane, heptane, octane, 19 decane, dodecane that doesn't break down, try to say that real quick, but you need to go down those twelve and you need to get them down in no time. here, because those are just the basic names, the rules will make this a little more complex quickly, well, let's take a look, so when we name alkanes, rule number one says to identify the longest continuous carbon chain, We call this Ching father, so if we look at In this first example here again, each vertex is a corner here in these line angle structures, so here we have 1 2 3 4 5 6 and we branch here and, to be I continue, I go up to the right or down.In this case, they are exactly equivalent, they are both methyl groups, 1 carbon chains from here on out and if I go one to the right or one down, it's the same thing, so you can choose your parent chain and then I'll just choose. down because most people would have chosen to the right. I'm going to choose down just for fun, but in this case this is just a 2 4 6 7 carbon chain, so we'll find out that it has the name ROO or at least we found out on the last slide, let's take a look at the one that's below here, so in this case I'll start from the right here if it's a little bit easier to see, so 1 2 3 4 5 so we get to this branch point I can go longer down here but I can also go longer up here and that's where this little special clause in rule number 1 says so if there are multiple ways to get to the longest chain then choose the one with the most substituents as the main chain now in this case if I continue to the left here, I would just have this substituent here coming off the main chain, but if I go up to this carbon and then to the right or to the left, then they are totally equivalent, so it doesn't really matter which one and in this case I will end up with two substituents and that's the number one rule that says it's the superior form, so I like to think that We're trying to make the longest word in the world and we'll do it if we have more substituents to name, as we'll see shortly, so let's move on to this one. from here to the right, so this one will be a little longer.
A little complicated here, but if we start with branch points, obviously it's wise to approach this systematically starting at these branch points and from these branch points I can go down to the left or I can go up and, as we just learned, exactly. In the last example, if we go up here and whether I go left or right at that point it doesn't matter, but if we go up here I would end up with two substituents, so that's definitely the way to go to more substituents instead of just one and as we go to the right, I get to this bifurcation point, if I go down, it's just two more carbons, if I go to the right, it's also just two more carbons, but I can see if I go to the right and if I go to some side. one of these three carbons are all equivalent, so I'll choose that one.
I could have chosen any of the three to be part of the main chain here, but I will get more substituents this way. I will get a total of three, how did we do it? We would have had a giant substituent on the right here, so note that we have one, two, three, four, five substituents, so keep mine if we had done it the wrong way, so maybe we would have just gone to carbon carbon carbon carbon carbon carbon carbon carbon and we thought, oh, go through the middle or something so you can see here, we only have one, two, three, four substituents and this is not the only wrong way, so we definitely have multiple ways to get the longest chain here but this one would only have four and again the correct one above has five substituents and that's a superior way for more substituents to give us a longer name and apparently I think that's the goal if we look , we have a main chain of 1 2 3 4 5 6 7 8 carbons that will be one octane as our main chain in this one, let's move on to the next rule, so now let's look at rule number two, so rule number 2 deals with the numbering of that continuous carbon chain, the main chain. here and it says you want to start at the end closest to the first substituent, your goal is to get that first substituent to the lowest possible number, so if we look at this first one here, I'm going to do it in blue, in this case if I number this of left to right 1 2 3 4 5 6 my first attachment my only substrate will be on carbon 6 but if I number it from right to left 1 2 3 4 5 6 and 7 I will place my substituent on carbon 2 of the main chain and that will be a superior way to number this if we do the same thing down here, so I'm going to give my main chain highlighted with red carbons here if I start from the right 1 2 3 4 5, the first substituent you see will be located on carbon 5, but if I go the other direction my first position will be located here on carbon 2 and that's the advantage of numbering through your main chain so last but not least we again cross this out as the wrong way to get the main chain, so with our correct form up here, if I number this from left to right, I'll get a substituent on carbon 2 here, so if I go from right to left I'll also get a substituent on carbon 2, so 1 2 , so they seem equivalent so far, but if I go from left to right 1 2 I get one substituent on carbon 3, and if I go from left to right again I get two substituents on carbon 2, so actually my second change It is also on carbon 2, and that is the key: if there is a tie with the first watchman, it is passed to the second substituent so that the second substituent has the lowest number and that is how we see it here, but it will actually work better if we go from right to left. so let's get rid of a couple of things there and number this from right to left and again there's our eight carbon chain.
Great, let's take a look at rule number three, so rule number three has this that identifies these substituents, so a substituent is anything that is not part of the main chain and in this case the carbon substituents will always end up with the yl suffix and they'll still use these same numerical designations that we've been using before here, so just with the yl suffix instead of an ane suffix so in this case, methyl ethyl propyl butyl pencil, hexa hepta loch lomond echo bass cabbage broth, etc., in this case, if I look at the first example here, this is simply a substituent on a carbon that will be a cool methyl group. and we see that also, if multiples are used, I try to repent of a hex, etc. to identify that there are multiple and we're also going to use the chain locator or the chain number, so Ana, before each of these fishermen, just to say where it's connected to. the main chain so here it is attached to carbon so as part of the name it will say: methyl find out separate the numbers from the letters so I go to the next example here this is also a carbon substituent which is a group methyl. so it's located on carbon two, so on that one we're also going to say two methyl and then this is an ethyl group and it's located on the main chain next to carbon three or on carbon three, so three: ethyl wood is part of the name there. and if we go to this one, we have a lot going on, so we have a couple of ethyl groups, so there are two of them located on carbon 3 and carbon 6, so since there are two, let's not just say ethyl, we'll say diethyl and we will give two string locators in this case 3 comma 6 - diethyl then - it still separates a number from a letter comma is going to separate the two numbers in this case, so three six diethyl will be part of the name so the other three substituents are all substituents on one carbon, so here they are all methyl groups, so here two of them located on carbon two, one located on carbon seven, since there are three of them, they will say that trimethyl is part of the name and if you say trimethyl here you have to give three chain locators, even if you have to repeat the chain locator, since two of them are attached to the same one, as is the case here, so here are two of them on carbon 2 and one in carbon. 7 so we'll say 2 comma 2 comma 7 - trimethyl as part of the name here as well.
Brilliant. I've had one more example here, so we still have to check the longest string and follow the rules here. so in this case, from this branch point where they are to the left or to the right, it doesn't matter, they are equivalent, we are going down the chain at this branch point, it is longer to go down to the right than to go up, so we'll go down here and from this point, if I go up to the right or down, it's equivalent, so I'm going to choose one of the two, it doesn't matter which one we can see, our longest chain is one, two, three. four five six seven carbons that's heptane again and then we can see all of our substituents we have one here one here and one here and in this case they are all methyl groups and having three of them it will say test and the name and in In this case, yes If we had a number, the longest string, if I numbered it from left to right, we would do this in blue.
I would have my first point one on carbon two, if I went from right to left, one two, my first request. I would also be on carbon two, but from left to right my next obstruent 153 four and it would end up being carbon five or as I can see if I go from right to left, that would also be my second change. I'm going to be in carbon. three, so let's take some of these and number them from right to left and then we can get the second one with the lowest number possible since the first substituent there was a numerical tie, so one, two, three, four, five, six and seven, and so with our trimethyl here they are located at two, three and six, so we'll say 2 3 point 6 - trimethyl again as part of the name, let's move on to the next rule, so now we're finally ready to name these things and We'll do that with rules number 4 and 5 here and see that they were supposed to raise the substituents in alphabetical order.
Note that their pre numeric like diet right behind them are not part of the alphabet just like methyl or ethyl or propyl below those that are female alphabetically only for the names of the substituents themselves, not the numerical prefixes, we'll find a couple of exceptions here, we also get your complex substituents here in a moment, for now this should work and in this case we are I'm going to use the Marana chain: we have the right diet, Tetris stuff already listed and stuff like that, so The first thing you say here is the substituents, so it's just 2 methyl, the only substituent I have, so then we're on.
I'm going to say the main chain and with a termination like we're supposed to do and for seven carbons it's heptane. Now this is the same word, there's no space between them, so it's just plain old heptane, so two methyl heptane for the first one here, so for the second one here we have 3 ethyl and two ethyl to say. Sorry, two methyl to say, but ethyl comes before methyl in the alphabet, sowe'll say that first, in this case 3 - ethyl - - - some of those hyphens always separate the letters from the numbers, so: methyl, and this is also a 7 carbon chain, so also heptane, again, no space here right into the word, we're trying to get the longest word in the world, so 3 ethyl 2 methyl heptane for the second one, let's look at the other two, so for the next one all we have is our methyl groups here , we already know that 2 point 3 point 6 - trimethyl is part of the name and in this case the only thing left to do Let's say it's all the substituents, is just the longest chain, which again is a 7 carbon chain, so which again there is no space and just old heptane, so 2 3 6 trimethyl heptane, so for the next one here we have two different sets of emissions. some methyls and Ethel and ethyl are first in the alphabet, so we'll say we'll first list these additions in alphabetical order, so these names will start with 3 comma 6 - diethyl and then - 2 comma 2 comma 7 - trimethyl great and our longest chain here is8 carbons and that is octane and again there is no space before the name of the main chains here, so this is 3 6 marked 2 - 7 trimethyl achtung let's look at a couple more examples, okay, and the next example.
I want to take a look at a special. case of rule number two, so rule number two here, the special case says that if all the substituents are located in the same locations in the chain, regardless of which way you number them, then we will find that the alphabet breaks the tie so let's look at our longest chain here it's the first one in this example so from this branch point we definitely need to go left and right instead of up both are longer than a carbon and we arrive at this bifurcation point, whether we go down to the right or up. the exact equivalent, so I'm going to choose one of the two and from here we can see that we have exactly two equivalents here, a methyl group and an ethyl group, so when we go to number this, we number it from left to right. two three so the first substituent that I find is on carbon 3 and if I go from right to left 1 2 3 the first substituent that I find is also on carbon 3 so I go to the second switch it was 1 2 3 from left to right 4 So, when we put that on top, so 4 5, our seconds on carbon 5, and if we go from right to left again, our second we would also see would be on carbon 5, so regardless of how we number this here, our substituents. are at 3 and 5 respectively, so in this case both directions produce exactly the same numbers and again the key here is for all the substituents, so the alphabet will break the tower here, so in this case the ethyl comes before the methyl in the alphabet and so we'll number it to give priority to the ethyl group, the lowest number and that happens, we go from right to left and it ends at carbon 3 here, so we'll continue through the chain and the method in blue here. is the preferred way to number this and so in this case we have to name this thing, we still name the substituents first, we'll still call Ethel before methyl since it's first in the alphabet and we list those substituents alphabetically, so what in this In this case we're going to say 3 ethyl and then 5 methyl and then our longest chain here 7 carbons which is heptane, so 3-ethyl 5-methyl heptane
If you have any copyright issue, please Contact