Electrons DO NOT Spin
Mar 10, 2024Quantum mechanics has a lot of strange things, but there is one thing that everyone agrees on and that no one understands. I am referring to quantum
spin
. Let's discover how chasing this elusive little behavior of the electron led us to some of the deepest insights into the nature of the quantum world. There is a classic demonstration performed in college physics courses: the physics teacher sits on a swivel stool and holds aspin
ning bicycle wheel. They turn the wheel and suddenly begin to spin on the chair. It is a demonstration of the conservation of angular momentum. The angular momentum of the wheel changes in one direction, so the professor's angular momentum has to increase in the other direction to leave the total angular momentum the same.Believe it or not, it's basically the same experiment: suspending an iron cylinder from a thread and activating a vertical magnetic field. The cylinder immediately begins to rotate with constant speed. At first glance, this seems to violate conservation of angular momentum because there was nothing spinning to begin with. Except there was, or at least in some ways there was. The external magnetic field magnetized the iron, causing the
electrons
in the iron's outer layers to align their spins. Thoseelectrons
act like little bicycle wheels and their displaced angular moments are compensated by the rotation of the cylinder. That explanation makes sense if we imagine that electrons spin like bicycle wheels or make anything spin.Which might sound good because electrons have this property we call spin. But there is a big problem: electrons definitely do NOT spin like bicycle wheels. And yet they seem to possess a very strange type of angular momentum that somehow exists without classical rotation. In fact, the spin of an electron is much more fundamental than simple rotation: it is a quantum property of particles, like mass or different charges. But it doesn't just make magnets move in strange ways: it turns out that quantum spin is a manifestation of a much deeper property of particles, a property that is responsible for the structure of all matter.
We'll unravel all of that in a couple of episodes, but today we'll talk about what spin actually is and get a little closer to understanding what this strange property of nature is. The iron cylinder experiment is called the Einstein de-Haas effect and was first performed by Einstein and de-Haas in 1915. It was not the first indication of the spin properties of electrons. This emerged by looking at the specific wavelengths of photons emitted when electrons jump between energy levels in atoms. Peiter Zeeman, working with the great Hendrik Lorenz in the Netherlands, discovered that these energy levels tend to split when atoms are placed in an external magnetic field.
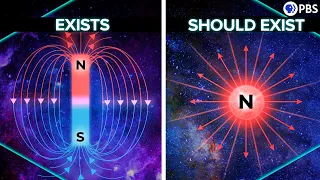
This Zeeman effect was explained by Lorentz himself with the ideas of classical physics. If you think of an electron as a ball of charge moving in circles around the atom, that motion leads to a magnetic moment: a dipole magnetic field like a small bar magnet. The different alignments of that orbital magnetic field in relation to the external field convert one energy level into three. Sounds reasonable. But then came the anomalous Zeeman effect. In some cases, the magnetic field causes the energy levels to split even further, for reasons that, at the time, were a complete mystery. One explanation that works is to say that each electron has its own magnetic moment: by itself it acts like a small bar magnet.
So the alignment of both the orbital magnetic moment and the electron's internal moment contributes to new energy levels. But for that to make sense, we really need to think of electrons as spinning balls of charge, but that poses huge problems. For example, to produce the observed magnetic moment, they would have to spin faster than the speed of light. This was first pointed out by the Austrian physicist Wolfgang Pauli. He showed that, if electrons are assumed to have a maximum possible size determined by the best measurements of the day, then their surfaces would have to move faster than light to provide the required angular momentum.
And that's assuming electrons even have a size (as far as we know, they're point-like), they have zero size, which would make the idea of classical angular momentum even more absurd. Pauli rejected the idea of associating such a classical property as rotation to the electron, insisting instead on calling it a "two classically non-describable values." Okay, so the electrons don't spin, but they somehow act as if they have angular momentum. And that's how we think about quantum spin now. It is an intrinsic angular momentum that influences the conservation of angular momentum as in the Einstein de-Haas effect, and also provides the electrons with a magnetic field.
The spin of an electron is an entirely quantum mechanical property and has all the oddities one would expect from the strangest theories. But before we dive into that weirdness, let me offer you one more experiment that reveals the magnetic properties that result from spin. This is the Stern-Gerlach experiment, proposed by Otto Stern in 1921 and carried out by Walther Gerlach a year later. In it, silver atoms are shot through a magnetic field with a gradient; in this example, stronger toward the north pole above and weaker downward. A lone electron in the outer shell of silver atoms gives the atom a magnetic moment.
That means that the external magnetic field induces a force on the atoms that depends on the direction these small magnetic moments are pointing relative to that field. Those that are perfectly aligned with the field will deviate the most, whether up or down. If these were classical dipole fields, like real small bar magnets, then those that were only partially aligned with the external field should deviate less. Thus, a stream of silver atoms with randomly aligned magnetic moments is sent through the magnetic field. You might expect a blurry patch of spots where the silver atoms hit the detector screen: some deflect up or down as much as possible, but most deflect somewhere in between because of all the random orientations.
But that is not what is observed. Instead, the atoms hit the screen at only two points corresponding to the most extreme deviations. Let's keep going. What happens if we remove the screen and put the beam of atoms back together? We now know that electrons must be aligned only up or down. Let's send them through a second set of Stern-Gerlach magnets, but now they are oriented horizontally. Classical dipoles that are 90 degrees from the field would experience no force. But if we place our detector screen, we see that the atoms land again on two points, now also oriented horizontally.
So not only do electrons have this rotationless magnetic moment, but the direction of the underlying magnetic moment is fundamentally quantum. The direction of this "spin" property is quantified: it can only take on specific values. And that direction depends on the direction you choose to measure it. Here we see an example of the two Pauli values manifesting as something like the direction of an axis of rotation or the north-south pole of the magnetic dipole. But in reality this double assessment is much deeper than that. To understand why we need to look at how spin is described in quantum mechanics.
Once again it was Pauli who achieved the first great success here. In the mid-1920s, physicists were very excited about a new tool they had been given: the Schrodinger equation. This equation describes how quantum objects behave as evolving probability distributions, like wave functions. It was proving to be surprisingly successful in describing some aspects of the subatomic world. But the equation as Schrodinger first conceived it did not include spin. Pauli managed to solve this problem by forcing the wave function to have two components, motivated by this ambiguous double value of the electrons. The wave function became a very strange mathematical object called spinor, which had been invented just a decade earlier.
And just a year after Pauli's discovery, Paul Dirac found his own even more complete solution to Schrodinger's equation, in this case to make it work with Einstein's special theory of relativity, something we've discussed before. Dirac wasn't even trying to incorporate spin, but the only way to derive the equation was by using spinors. Now, spinors are exceptionally weird and cool, and they really deserve their own episode. But let me tell you a couple of things to give you an idea. They describe particles that have very strange rotation properties. For familiar objects, a 360-degree rotation returns them to their starting point.
This also applies to vectors, which are simply arrows pointing in some space. But for a spinor it is necessary to rotate it twice (or 720 degrees) to return to its initial state. Below is an example of spinor-like behavior. If I turn this cup without letting go, my arm twists. A second rotation untangles me. We can also visualize this with a cube attached to nearby walls with ribbons. If we rotate the cube 360 degrees, the cube itself returns to the starting point, but the ribbons have a twist compared to how they started. Surprisingly, if we turn another 360º (not backwards but in the same direction), the entire system returns to its original state.
Another thing to note is that the cube can spin any number of times, with any number of ribbons attached, and never gets tangled. So think of electrons as being connected to every other point in the universe by invisible threads. One rotation causes a spin, two returns it to normal. To be a little more technical, the spinor wavefunction has a phase that changes with orientation angle and a 360 degree rotation puts it out of phase compared to its initial point. To get an idea of what spin really is, don't think about angular momentum, but rather regular or linear momentum.
The momentum of a particle is fundamentally related to its position. According to Noter's theorem, the invariance of the laws of motion under changes in the location of coordinates gives us the law of conservation of momentum. For related reasons, in quantum mechanics position and momentum are conjugate variables. Which means you can represent a particle wave function in terms of any of these properties. And according to Heisenberg's uncertainty principle, increasing the knowledge of one means increasing the unknowability of the other. If position is the companion variable of momentum, what is the companion variable of angular momentum? Well, it's angular position.
In other words, the orientation of the particle. So one way to think about the angular momentum of an electron is not from classical rotation, but rather from the fact that they have a rotational degree of freedom that leads to a conserved quantity associated with that. They have an indefinite orientation, but a perfectly defined angular momentum. Some physicists think that spin is more physical than this. Han Ohanian, author of one of the most widely used quantum textbooks. shows that the correct values of the angular momentum and magnetic moment of the electron spin can be derived by observing the energy and charge currents in the so-called Dirac field.
That is the quantum field surrounding the Dirac spinor, also known as the electron, which implies that even if the electron is punctual, its angular momentum can arise from an extended but still small region. Regardless of how you explain it, we have a great working definition of how the effect works. We say that the particles described by the spinors have spin quantum numbers that are half-integers: ½, 3/2, 5/2, etc. The electron itself has spin ½, just like the proton and neutron. Their intrinsic angular moments can only be observed as about half of the reduced Planck constant, projected in whatever direction one attempts to measure.
We call these particles fermions. Particles that have integer spin (0, 1, 2, etc.) are called bosons and include force-carrying particles such as photons, gluons, etc. These are not described by spinors but by vectors, and behave in a more intuitive way. 360 degree rotation returns them to their original state. This difference in the rotational properties of fermions and bosons results in profound differences in their behavior: it defines how they interact with each other. Bosons, for example, can accumulate in the same quantum states, while fermions can never occupy the same state. This antisocial behavior of fermions manifests itself as the Pauli Exclusion Principle and is responsible for us having a periodic table, for electrons living in their own energy levels, and for thematter really has structure.
It's the reason you're not falling off the ground right now. But why should this obscure rotational property lead to such fundamental behavior? Well, this is all part of what we call the spin statistics theorem, which we'll come back to in an episode very soon. Electrons aren't spinning: they're doing something much more interesting. What we call spin is a clue to the structure of matter, and perhaps the structure of reality itself through these things we call spinors, strange little knots in the subatomic fabric of space-time. Last time we talked about the connection between quantum entanglement and entropy, this was a heady topic, to say the least, but you guys had incredibly insightful comments and questions.
Joseph Paul Duffey asks whether entropy is an illusion created by our observation of isolated components within a "larger" entangled system. Well, the answer is that entropy is relative. It is high or low depending on the context. The air in a room can be perfectly mixed and therefore considered “high” entropy. But if that room is warm compared to a cold environment outside, then the total room + environment has a relatively low entropy compared to the maximum, if you opened the doors and let the temperature equalize. Von Neumann entropy is different from thermodynamic entropy in the sense that it represents the information contained in the system and extractable in principle, versus the information that the system loses when intertwined with the environment.
On the other hand, classical or thermodynamic entropy represents information that is hidden under the raw properties of the system, but that in principle can be extracted. And yet von Neumann entropy has a similar contextual nature. If your system is not entangled with the environment, then its von Neumann entropy is zero. But if we consider a subsystem within that system, then that entropy increases. Randomaited asks the following: If entropy only increased with time, implying that it was at its minimum at the Big Bang, does that mean there was no quantum entanglement at the Big Bang? To answer this we would need to know why entropy is so low at the Big Bang, and that is one of the central mysteries of the universe.
But I'll try anyway. So we can't really talk about the beginning of time t=0, because that moment was lost in our ignorance about quantum gravity and inflation and every other crazy theory we haven't discovered yet. But what we do know is that in a very, very small period of time after t=0, the universe was extremely compact, which meant hot and dense, and it was also extremely smooth. The compact part is where the low entropy comes from. The “gravitational degrees of freedom” were almost completely unoccupied. On the other hand, the extreme softness meant that the entropy associated with the matter was extremely high.
The energy was as dispersed as possible between all the particles and the different ways they could move. The low gravitational entropy greatly exceeded the entropy of matter, so the entropy was low. That softness seems to suggest that the particles of the early universe were already entangled; Otherwise, how did they distribute their energy? Chris Hansen makes the same point, asking if the conditions of the Big Bang meant that everything started in an intertwined way. You might think so, but that's not necessarily the case. Remember that von Neumann entropy is relative to the system you are talking about, as is entanglement.
Let's say you have a bunch of particles that are not entangled with each other, but with another bunch of particles somewhere else. If you ignore those other particles, then it appears that there is no entanglement in the particles of the first system. And yet, those particles can have correlated thermodynamic properties due to their mutual connection to the outside. In the early universe, the extreme expansion of cosmic inflation may have permanently separated entangled regions, but left those regions with an internal thermal balance that does NOT require maximum entanglement within the regions themselves. In other words, the universe—or our portion of it—may have started out unentangled and with low entropy, even if it was in thermal equilibrium.
Lincoln Mwangi also revealed some insights, informing us that "The Cloud" - is actually named after Dr. Shannon, the founder of the field of information theory. As with many of these things, the word has become corrupted over time and is now commonly mispronounced. This is very disrespectful and I intend to write a series of opinion pieces to correct the matter. Right after we uploaded this video to the Claude.
If you have any copyright issue, please Contact