Chemical Equations | Environmental Chemistry | Chemistry | FuseSchool
Feb 23, 2020Chemical
equations
can sometimes seem a little daunting, especially when there are many different compounds and state symbols involved, but don't be afraid to help in this lesson onchemical
equations
. Simply put, achemical
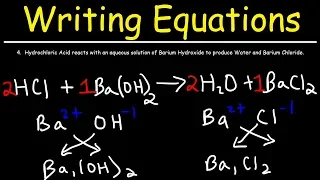
equation shows the overall chemical change of reactants into products. It's a bit like a detailed cooking recipe but where all the ingredients and all the products are written down, even the ones that you can't necessarily see, the reactants are what you start with and the products are what you form. There are two ways to write chemical equations, word equations and symbols. Equations when written, both types show the reactants to the left of an arrow and the products to the right.The arrow is there to show that the reaction is irreversible, if you like, it shows the direction of the reaction and that it is unidirectional. a bit like a one-way street that cannot be reversed along a one-way street and an irreversible reaction cannot be reversed. Let's look at a verbal equation. This is a way of summarizing a chemical reaction, for example the neutralization of hydrochloric acid. with sodium hydroxide we would write sodium hydroxide plus hydrochloric acid then the arrow sodium chloride plus water the plus sign indicates that there is more than one reactant or product on each side of the equation and you will notice that the reactants are on the left and the products are on the right.
We've also written all of this on one line, which makes it much easier to read this way, so when writing your equations try to keep all the information on one line if you can't. the arrow becomes an important separator the rule of reactants on the left and products on the right still applies because if not, the equation can become a mess a clearly written equation is always easier to understand an equation with words provides a good summary, but a symbol equation provides more information, shows more detail, and allows us to see how many atoms and molecules are involved in each reaction, so if we look at the neutralization equation again, the symbol equation is written naoh more hcl as the reactants and then an arrow to indicate the irreversible reaction. which gives nacl plus h2o as products the lowercase letters in parentheses are state symbols, they show the state of matter of each product and reactant and this is covered in more detail in our lesson state symbols in chemical equations there are times when A reaction is reversible due to changes in the environment, for example pressure, concentration, pH and temperature, and when this is the case, we draw a double arrow made up of two half arrows pointing in opposite directions and this indicates that the reaction can go in either direction, the formation of ammonia from nitrogen. and hydrogen is an example of a reversible reaction and the key here is that you recognize the reversible reaction by the double arrows and you will write down some numbers in front of the hydrogen and ammonia formulas and these are there to make the equation balance for you.
You can find more information about this in our balancing equations lesson and to summarize, a chemical equation shows the general chemical change of reactants to products, we usually write reactants to the left of the arrow and products to the right. A single arrow means the reaction is irreversible and two half arrows pointing opposite mean the reaction is reversible and that completes our overview of the chemical equations that
If you have any copyright issue, please Contact





![[[Short MunawarTalks]] Which Arabic Course to select ?](https://i.ytimg.com/vi_webp/aGkP2-p649U/mqdefault.webp)