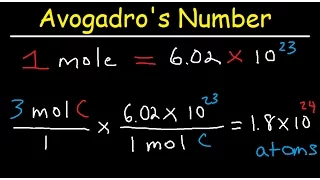
Calculate the Mass of a Single Atom or Molecule
Feb 23, 2020In this video we are going to learn how to
calculate
themass
of asingle
atom
ormolecule
. What is themass
in grams of asingle
silveratom
? To solve this problem we are going to have to pull out a few different pieces of information about moles, the first one will be about mass because here we are talking about the mass of silver atoms, so what do we know about the mass of silver? Well, we can look it up on the periodic table at this number here, 107.9 tells us. The molar mass of silver tells us how much one mole of silver weighs in grams, so we can say that one mole of silver atoms weighs one hundred seven point nine grams, well that's what we know about the mass of silver that we don't know .Here we are talking about a mole of silver, although we are talking about a single atom of silver, so what do we know about the relationship between moles and the number of things in a mole? Well, we know that one mole of silver atoms contains 602 hexillion. sulfur atoms and this is often abbreviated as 6.02 times 10 to the 23 silver atoms. Now, to solve this problem, we want to take these two pieces of information and combine them to form a third piece, which will be the most useful force. Well, here are the Two things we know well: we know that one mole of silver atoms weighs that much and we also know how many silver atoms (602 hexillion) there are in one mole, so from these two pieces of information we can say that 602 hexillion of silver atoms in 1 mole are 107.9 grams.
Well, now we know how much this number of silver atoms weighs. Well, we can even write this as a kind of equation, a relationship and equivalence, and from this relationship we can write two conversion factors, one of these has grams on the top and the other has grams on the bottom, but both can allow us to convert back and forth between the number of silver atoms we have and their mass in grams, so we work with this data and we can write this equation which then allows us to write two conversion factors that allow us to go from the number of silver atoms to grams, we'll use one of these conversion factors to go from a single silver atom to how much it would weigh in grams, so let's see how we'll start, okay?
We're going to do a conversion factor problem where we start with a silver atom, an application of silver, and we're going to want to multiply a silver atom by one of these two conversion factors and the one we're going to choose is going to be the which is going to cancel out the silver atoms which is at the top of this side of the equation, okay, so it's at the top here, we're going to want it at the bottom, so this one won't work because the silver atoms silver are at the top, but this is when we want, okay, so the silver atoms at the top cancel the silver atoms at the bottom cancel and it will leave us with grams.
Now that we've written this down, we can solve it with a calculator. If you write it like this, we'll get this as our answer, which is just the way the calculator says this number multiplied by 10 to the power of negative 22 and all we have to do now is round this using significant figures because calculators don't do that. . around this we're going to round this to three significant figures, we don't worry about the number of significant figures in the one because it's a counting number, it's not a measurement, so we're going to do one point seven nine times ten to the twentieth second negative what are the units here are grams and that's our final answer for the mass of a single atom of silver and just to show you that this is not an exponential notation here is a decimal point and up to here a seven nine grams this is a Tiny, tiny number is how much a single atom of silver weighs.
Now let's look at how to do this with
molecule
s instead of just atoms. What is the mass in grams of a single water molecule? This is a lot like calculating. the mass of a single atom, except that's just a couple of extra things we need to take into account, so here's a water molecule h2o that's made up of two hydrogen atoms and one oxygen atom. The first thing we want to do iscalculate
the molar mass of h2o, okay, we want to know how much a mole of h2o molecules would weigh in grams, okay, to do this it is composed of hydrogen and oxygen, so we look up hydrogen and oxygen in the periodic table , here is a molar mass of hydrogen, here is the molar mass. of oxygen and let's add them together, so there are two hydrogen atoms in the water, so we do twice the molar mass of hydrogen and then there is one oxygen atom, so we do once the molar mass of oxygen, we add this. and we get eighteen point zero two, which tells us the molar mass and tells us that one mole of h2o molecules weighs eighteen point zero two grams.Now we know how much one mole of h2o molecules weighs so we can make conversions between the number of molecules. and mass, we need to know a little bit about the amount of stuff in a mole, which is 602 hexillions of h2o molecules in one mole of h2o molecules, and like we did before with silver, we can combine these two pieces of information to say that 602 hexillions of h2o. molecules is one mole and weighs eighteen point zero two grams. We can express this as a ratio and we can use this to write two conversion factors.
They are both based on this equation. It's just that one is the inverted version of the other. Okay, so I have these two conversion factors that we can write from this information here and this is how we're going to use them. Well, we're starting with a molecule of water and what I want to do is multiply it by the conversion factor that will remove the water. molecules, so I'm going to want to use the one with h2o here at the bottom so the h2o molecules cancel out. The h2o molecules cancel out there, it will leave me with grams and when I check and do the calculations, round to three significant figures.
I'm going to end with two point nine nine times ten to the power of negative twenty-three grams. This is how you would plug it into your calculator and the answer you would get and finally, I always like to emphasize that this number here is not just some weird Martian number with this 10 to the power of negative 23, but it's actually a very, very small that, if you have time, could be written with many, many, many, many zeros, a tiny lowercase. quantity in grams the mass of a single water molecule h2o
If you have any copyright issue, please Contact